Abstract
The backbone and side chain resonance assignments of the periplasmic domain of HasB, the energy transducer for heme active transport through the specific receptor HasR of Serratia marcescens, have been determined as a first step towards its structural study. The BMRB accession code is 15440.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Biological context
In addition to an inner membrane, Gram-negative bacteria have an outer membrane that affords additional environmental protection to the organism. This outer membrane is a selective permeation barrier. Various molecules, including iron, ferric siderophores, vitamin B12 and heme are too large or usually present at a concentration too low to diffuse through the outer membrane pores. Consequently, specific surface receptors promote the translocation of these various substrates by an energized mechanism. This energy-dependent transport is mediated by a cytoplasmic membrane complex consisting of three proteins: TonB, ExbB and ExbD (Postle 1993; Larsen et al. 1996). Current models consider TonB to function as the energy transducer that couples the proton motive force of the cytoplasmic membrane to drive ligand translocation through the outer membrane receptors. TonB is a three-domain protein containing an N-terminal transmembrane helix that anchors the protein in the cytoplasmic membrane, a central proline-rich domain that resides within the periplasm and a C-terminal globular domain that directly contacts outer membrane receptors. Two structures of the C-terminal domain of TonB complexed with an outer membrane receptor are now available (Pawelek et al. 2006; Shultis et al. 2006).
In many species, there is only one TonB protein that is shared by the various outer membrane receptors. However, some bacteria species contain several different copies of distinct genes for TonB homologs in their genome. In addition to the TonB protein, the Gram-negative bacteria Serratia marcescens possesses an additional TonB-like protein named HasB. This protein shares about 20% identity with the E. coli and the S. marcesens TonB proteins and has the same structural organisation than that of TonB. The S. marcesens TonB is active for a broad spectrum of TonB-dependent functions whereas HasB is only involved in heme uptake through the specific receptor HasR (Paquelin et al. 2001). The basis of this specificity is unknown. The most likely explanation for this specificity can come from structural differences between the C-terminal domains of the two proteins, TonB and HasB, leading to differences in the interaction with the receptor HasR.
The 1H, 15N and 13C backbone and side chain resonance assignments of the periplasmic domain corresponding to the HasB 131 C-terminal residues have been determined as a first step towards the structure determination. This is the first structural study of a specific TonB-like protein.
Methods and experiments
The C-terminal domain of HasB (HasB133) comprises the last 131 residues of HasB (residues 133–263) and an additional N-terminal methionine. The construction has no tag nor signal peptide. The cDNA fragment encoding HasB133 was synthesized by PCR from the plasmid pHasBpuc (Paquelin et al. 2001) and cloned into a pBAD24 vector. E. coli JP313 cells (Economou et al. 1995) harbouring the plasmid coding for HasB133 were grown at 37°C in a 1.4 l bioreactor of stable-isotopically labelled M9 minimal medium containing 1 g/l 15N NH4Cl and 4 g/l 13C glycerol as the sole nitrogen and carbon sources respectively, and complemented with 0.5 mg/l thiamine, 6 μM FeSO4 and 6 μM sodium citrate. Protein expression was induced from the start of the culture with 0.2 g/l l-arabinose and incubation was continued at 37°C for 8 h until OD600nm reaches 5.0. Wet cells were then disrupted in a French press in the buffer A (50 mM Tris–HCl, 100 mM NaCl, pH 8.5). Clarified cell lysate was then loaded on a HiLoad 16/10 SP Sepharose cation-exchange column (GE Healthcare Life Science) equilibrated with buffer A. The protein was eluted with 20 column volumes of a linear gradient of buffer A to 100% of buffer B (50 mM Tris–HCl, 1M NaCl, pH 8.5) at a flow rate of 2 ml/min. The samples containing HasB133 were combined and concentrated by ultrafiltration (Amicon 5 kDa cutoff) to 1 ml and loaded onto a size exclusion column (Sephacryl S-100 HP 16/60) equilibrated with buffer C (50 mM sodium phosphate, 50 mM NaCl, pH 7). Finally the pure samples were combined and concentrated to 0.8 mM in the buffer C with H2O/D2O (85/15 v/v). The protein concentration was estimated from its absorbance at 280 nm assuming a calculated ε280 of 10,000 M−1 cm−1. All the purification steps were performed at +4°C and in presence of a protease inhibitor cocktail (Roche).
All NMR experiments were recorded at 293 K on Varian spectrometer operating at a proton frequency of 600 MHz and equipped with a cryogenically-cooled triple resonance 1H (13C/15N) PFG probe. The sequence specific 1HN, 15N, Cα and C′ backbone resonances assignments were based on the following experiments: 15N–1H HSQC, 3D HNCO, 3D CBCA(CO)NH & 3D HNCACB. The side chain 1H, 15N and 13C resonances were manually assigned using 3D H(CCO)NH, 3D 1H–15N HSQC-TOCSY and 3D C(CO)NH experiments. Assignments of aromatic amino acids were obtained with the 2D 1H–13C HSQC, 2D CB(CGCD)HD and 2D CB(CGCDCE)HE experiments. DSS was used as direct 1H chemical shifts reference and indirect reference for 15N and 13C chemical shifts (Wishart et al. 1995). The pulse sequences of experiments were taken as implemented from the Varian Biopack (http://www.varianinc.com). The spectra were processed with NMRPipe (Delaglio et al. 1995) and analysed with the XEASY program (Bartels et al. 1995).
Assignments and data deposition
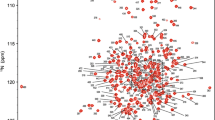
High-quality NMR data for HasB133 were obtained, as illustrated by the 15N–1H-HSQC spectrum shown in Fig. 1. Backbone assignments were obtained for all non-proline residues except the 1HN and N of Lys2, Asn36 and Ile 41. The region 34–42 seems to undergo conformational or solvent exchange, since the signals are unusually weak. The C′ are missing for all residues preceding the 12 prolines present in HasB133. 1H, 13C chemical shifts were obtained for 86% of the CHn and aromatic side chains. Assignments of γ15NH2 of two out of the three Asn and seven out of the eight Gln residues are reported. The chemical shifts have been deposited in the BioMagResBank (http://www.bmrb.wisc.edu) with the accession number 15440.
The comparison of the localisation of the secondary structure elements of the C-terminal region of HasB (http://www.marlin.bmrb.wisc.edu/devise/peptide-cgi/html/15440c1.html) with that of equivalent region of E. coli TonB (PDBID: 1xx3) reveals some differences in N and C-terminal extremities. Two helices H1 and H4 are not observed in TonB, the last β strand of TonB is not present in HasB (Fig. 2).
Secondary-structure elements of the C-terminal domain of HasB deduced from the consensus Chemical Shift Index (http://www.marlin.bmrb.wisc.edu/devise/peptide-cgi/html/15440c1.html) compared with that of the equivalent region of TonB (PDB ID: 1xx3). Asterisks indicate conserved residues in two proteins. Arrows represent the β-strands and cylinders the helices
References
Bartels Ch, Xia TH, Billeter M, Güntert P, Wüthrich K (1995) The program XEASY for computer-supported NMR spectral analysis of biological macromolecules. J Biomol NMR 5:1–10
Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6:277–293
Economou A, Pogliano JA, Beckwith J, Oliver DB, Wickner W (1995) SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell 83:1171–1181
Larsen RA, Myers PS, Skare JT, Seachord CL, Darveau RP, Postle K (1996) Identification of TonB homologs in the family Enterobacteriaceae and evidence for conservation of TonB-dependent energy transduction complexes. J Bacteriol 178:1363–1373
Paquelin A, Ghigo JM, Bertin S, Wandersman C (2001) Characterization of HasB, a Serratia marcescens TonB-like protein specifically involved in the haemophore-dependent haem acquisition system. Mol Microbiol 42:995–1005
Pawelek PD, Croteau N, Ng-Thow-Hing C, Khursigara CM, Moiseeva N, Allaire M, Coulton JW (2006) Structure of TonB in complex with FhuA, E. coli outer membrane receptor. Science 312(5778):1399–1402
Postle K (1993) TonB protein and energy transduction between membranes. J Bioenerg Biomembr 25:591–601
Shultis DD, Purdy MD, Banchs CN, Wiener MC (2006) Outer membrane active transport: structure of the BtuB:TonB complex. Science 312(5778):1396–1399
Wishart DS, Bigam CG, Yao J, Abildgraad F, Dyson HJ, Oldfield E, Markley JL, Sykes BD (1995) 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J Biomol NMR 6:135–140
Acknowledgements
We thank Cécile Wandersman for constant interest in this work. Julien Lefèvre was supported by a fellowship of the Ministère de l’Education Nationale, de la Recherche et de la Technologie (MENRT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lefèvre, J., Simenel, C., Delepelaire, P. et al. 1H, 13C and 15N resonance assignments of the C-terminal domain of HasB, a specific TonB like protein, from Serratia marcescens . Biomol NMR Assign 1, 197–199 (2007). https://doi.org/10.1007/s12104-007-9055-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-007-9055-7