Abstract
In the past decade, therapeutic hypothermia using a variety of low-cost devices has been widely implemented in India and other low-and middle-income countries (LMIC) without adequate evidence of either safety or efficacy. The recently reported data from the world’s largest cooling trial (HELIX - hypothermia for encephalopathy in low- and middle-income countries) in LMIC provides definitive evidence of harm of cooling therapy with increase in mortality (number to harm 9) and lack of neuroprotection. Although the HELIX participating centers were highly selected tertiary neonatal intensive care units in South Asia with facilities for invasive ventilation, cardiovascular support, and 3 Tesla magnetic resonance imaging (MRI), and the trial used state-of-the-art automated servo-controlled cooling devices, a therapy that is harmful under such optimal conditions cannot be safe in low-resource settings that cannot even afford servo-controlled cooling devices.
The HELIX trial has set a new benchmark for conducting high quality randomized controlled trials in terms of research governance, consent, ethics, follow-up rates, and involvement of parents. The standard care for neonatal encephalopathy in LMIC should remain normothermia, with close attention to prevention of hyperthermia. There is no role for therapeutic hypothermia in LMIC as the efficacy of hypothermia is dependent on the population, and not merely on the level of neonatal intensive care facilities. Future research should explore timings and origins of brain injury and prevention of brain injury in LMIC, with a strong emphasis on academic research capacity building and patient and public engagement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neonatal encephalopathy is the most common cause of death and serious brain injury in term infants affecting at least 1.2 million infants globally every year; over 96% occurs in low- and middle-income countries (LMICs) [1]. In the United Kingdom and most high-income countries, therapeutic hypothermia has been the standard therapy for neonatal encephalopathy since 2007 and is recommended by the International Liaison Committee on Resuscitation (ILCOR) guidelines [2]. Although there was compelling evidence why the safety and efficacy of cooling therapy from high-income countries could not be directly extrapolated to LMICs, it was widely introduced into the clinical practice in India and other low-resource settings without adequate evaluation [3, 4].
The recently reported data from the largest cooling trial in the world, and the only phase III clinical trial in LMIC (HELIX trial - hypothermia for encephalopathy in low- and middle-income countries), provides definitive evidence of harm—therapeutic hypothermia increases death; one additional infant will die for every 9 treated infants [5]. Assuming a minimal case scenario of 8% eligible encephalopathic infants currently receive hypothermia, an additional 25 deaths are expected to be occurring from cooling therapy every single day in LMIC. These deaths could be prevented purely by discontinuing the use of therapeutic hypothermia in these settings.
The data from the HELIX trial not only highlights the dangers of clinical implementation without rigorous evaluation in clinical trials, but also challenges the current understanding about the origins and nature of brain injury after neonatal encephalopathy in LMIC [5]. In this review, the authors discuss how and why cooling therapy was implemented in India and other LMIC without adequate evidence, explore the challenges in conducting high-quality research in LMIC, and provide some suggestions for future research.
Reasons for Therapeutic Drift of Cooling in LMIC
False Reassurances from Observational Studies
While observational studies and registries are useful to evaluate how well a particular treatment is implemented, they should never be used to examine the safety and efficacy of a new treatment. A large number of observational studies on cooling therapy have been reported from India and other LMICs, all reporting good outcomes [6,7,8]. However, without an appropriate control arm, these data make clinicians no wiser regarding the safety or efficacy of hypothermia. Infants with perinatal asphyxia, with no or mild encephalopathy, are likely to do well without any treatment [9]. It is very likely that the studies reporting good outcomes after therapeutic hypothermia had included babies with mild or even no encephalopathy.
Pilot Randomized Controlled Trials and Poor Trial Designs
To date, 14 single-center pilot randomized controlled trials and one phase III multicenter clinical trial have been reported from LMIC. Although none of the pilot trials were powered to look at any clinical outcomes, increased mortality during the intervention was seen in three studies [10,11,12] (Table 1 and Fig. 1).
Meta-analysis of all the pilot cooling trials prior to the HELIX trial, showing significant reduction in neonatal mortality with therapeutic hypothermia. Given the suboptimal quality of the individual studies and possible duplicate publications, it is unlikely that these pooled data are meaningful. (References—Akisu et al. [41], Lin et al. [40], Bhatt [39], Robertson et al. [11], Zhou et al. [15], Bharadwaj and Bhat [38], Thayyil et al. [10], Joy et al. [37], Shimi et al. [36], Gane et al. [35], Tanigasalam et al. [34], Rakesh et al. [33], Chen et al. [32], Catherine et al. [31], Thomas et al. [6])
In 2007, Robertson et al. conducted a pilot cooling trial using water bottles filled with tap water in a sub-Saharan neonatal unit [11]. The unit lacked basic neonatal care facilities, including radiant warmers or oxygen saturation monitors, let alone facilities for ventilatory or inotropic support, and not surprisingly mortality was 6 times higher in the cooled infants. Although these investigators argued that the high mortality was due to coexistent sepsis rather than lack of supportive care during cooling therapy, subsequent detailed infection studies from the same hospital showed coexistent sepsis was present in less than 9% of the infants with encephalopathy [13]; a similar incidence as in high-income countries [14].
In 2009, Thayyil et al. reported a pilot cooling trial from Calicut Medical College, Kerala, India involving 33 infants with encephalopathy, using phase change material. Mortality in the cooled infants were twice (24%) that as usual care (13%) infants [10]. Therapeutic hypothermia did not improve whole brain fractional anisotropy; however, the trial was not powered to examine magnetic resonance biomarkers.
In 2020, Aker et al. reported a pilot study (THIN study) from Christian Medical College, Vellore, again using phase change mattress. Mortality during the intervention was twice (8%) in the cooled infants when compared with usual care (4%) [12]. The low mortality in the usual care infants may reflect inadvertent inclusion of infants with mild encephalopathy due to poor standardization of the clinical examination. Although investigators intended to use MR biomarkers to measure treatment effects, this could be obtained only in 22 infants, and hence no meaningful conclusions could be made from this study. Nevertheless, authors made a bold statement on safety and efficacy of cooling based on this very small pilot study, reflecting a lack of understanding of the complexity of using MR biomarkers in neuroprotection trials.
Between 2012 and 2020, six randomized controlled trials were reported from one Indian hospital—Jawaharlal Institute of Postgraduate Medical Education and Research, Pondicherry. Despite similar inclusion criteria, mortality varied between 7% and 50% in the usual care group in these six trials, raising concerns about the reliability of these data.
The only phase III clinical trial was reported from China in 2010 and involved selective head cooling using a locally developed device. A total of 235 infants were recruited; however, the large number of postrandomization exclusions, high loss to follow-up, and protocol violations made the trial results unreliable [15]. Thus, although the pooled data from the LMIC trials show a reduction in neonatal mortality, these data have little scientific relevance due to poor trial quality of the individual studies (Fig. 1) [16].
Commercialization of Cooling Therapy in LMIC
In theory, any device that maintains core temperature within a narrow target range is likely to be effective in administering therapeutic hypothermia. However, manual techniques are likely to result in excessive temperature fluctuations and uncontrolled rewarming, unless used alongside 1:1 nursing care. The latter is more expensive in LMIC than servo-controlled cooling devices.
Phase change materials (PCM) offer an alternative to cooling with ice. PCM are made of polymers with long-chain molecules primarily of carbon and hydrogen. During melting and freezing, the material releases thermal energy. The first ever clinical study of phase change material for neonatal hypothermia was conducted at Calicut Medical College, Kerala, India in 2009 [10]. Although the phase change cooling mattress was inexpensive (Rs 10,000), the cooling efficacy was dependent on the ambient temperature, induction was slow, and re-warming was uncontrolled.
Despite these concerns, Thomas and colleagues from Christian Medical College Vellore marketed phase change material in India (Miracradle; Rs 1.6 Lakh [17] with claims of it being a lifesaving and innovative device. Unfortunately, this has led to many hospitals in India implementing therapeutic hypothermia using phase change material, before the HELIX trial was reported.
Imperial College London team, in collaboration with Inspiration Health Care (UK), developed a servo-controlled cooling device (Tecotherm HELIX) with funding from the Gates foundation [18]. This was an automated servo-controlled method suitable for LMIC, with an estimated manufacturing cost of under Rs 1 Lakh. However, the marketing of the device was kept on hold until the safety of cooling therapy was established in LMIC, to avoid potential harm to babies.
Ignoring Population Differences and Misleading Infection–Ischemia Theory
The discussions around extrapolating the evidence from cooling therapy from high-income countries to LMICs were primarily focussed on developing cooling devices and supportive intensive care, rather than on population differences [19]. Even the Cochrane meta-analysis [20] and the ILCOR guidelines overlooked the importance of population differences, and recommended cooling therapy in LMIC neonatal units, where adequate resources to offer intravenous therapy, respiratory support, pulse oximetry, antibiotics, anticonvulsants, and pathology testing are available.
Meanwhile, some researchers proposed infection was the main cause of encephalopathy in African settings. More recent data from Africa [13, 21] and South Asia suggest that the incidence of coexistent sepsis in Africa and South Asia is not higher than high-income countries [14] and coexistent sepsis cannot explain lack of hypothermic neuroprotection in LMIC.
Hence, the population differences between high-income countries and LMIC appear to be far more complex and multifactorial; attributing all this to coexistent sepsis appears rather naïve. The differences in socioeconomic factors in brain injury are increasingly recognized even within high-income countries, where these factors have a major effect on the developing brain.
Current Practice of Therapeutic Hypothermia in India and Other LMIC
A national survey done in 93 tertiary Indian neonatal units in 2015 reported that 51% offered cooling therapy and further 44% wanted to offer cooling therapy, but were unable to do so due to a lack of cooling devices [22]. A prospective cross-sectional national survey involving 1092 professionals in Brazil reported that 62% were routinely cooling babies. Of these, 29% were even cooling infants with mild encephalopathy [23]. Another web-based survey involving 288 paediatricians and neonatologists in South Africa reported that 76% of the respondents firmly believed therapeutic hypothermia would be beneficial and 46% had been already cooling encephalopathic neonates in their units [24]. Wide implementation of therapeutic hypothermia across neonatal units in Morocco was suggested based on the outcome data of a prospective study involving just 32 asphyxiated neonates in a tertiary hospital in Marakkech [25].
The HELIX Trial
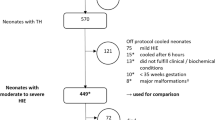
In 2010, the HELIX trial consortium was established to conduct high-quality clinical trials in neonatal encephalopathy in collaboration with Imperial College London, and involved several tertiary neonatal units in South Asia. A HELIX feasibility trial was conducted initially at the consortium sites and included 82 babies with moderate to severe encephalopathy. Sixty-one babies (74%) had moderate and 21 (26%) had severe encephalopathy. The neonatal mortality was 29% in the cooled infants as opposed to 54% in a contemporary of 112 noncooled infants, highlighting the effect of selection bias in nonrandomized trials [18].
A final consortium of seven tertiary neonatal units in South India, Sri Lanka, and Bangladesh was then identified based on the quality of the research data and access to advanced neuroimaging. All HELIX cooling centers had adequate research governance to conduct credible clinical trials and included Sion Medical College, Mumbai; Indira Gandhi Institute of Child Health, Bengaluru; Bangalore Medical College, Bengaluru; Madras Medical College, Chennai; Trivandrum Medical College, Thiruvananthapuram; Bangabandhu Sheikh Mujib Medical University (BMMSU), Dhaka and Kelaniya University, Sri Lanka. These centers were well-resourced tertiary intensive care units with excellent facilities for invasive ventilation including high-frequency oscillation, cardiovascular monitoring and support, and access to 3 Tesla MR imaging and spectroscopy [5] (Fig. 2).
Thus, after five years of preliminary work, a phase III randomized controlled trial of whole-body cooling versus usual care (control group) was started in August 2015. Dedicated research nurses and fellows were appointed for the trial, and the trial was closely monitored and supported by an experienced neonatal neurology team from Imperial College London. A state-of-the-art and CE marked automated servo-controlled cooling device (Tecotherm Neo) administered cooling therapy.
The HELIX trial included 408 infants; of which, 202 were randomized to hypothermia group and 206 to the usual care group. The primary outcome data (death or moderate or severe disability) at 18 mo were available for 394 (96.5%) infants [5]. Therapeutic hypothermia did not reduce the combined rate of death or moderate or severe neurodisability at 18 mo (98 babies (50.3%) of the hypothermia and 94 (47.2%) of the usual care group [Risk Ratio (RR) 1.06; 95% CI 0.87 to 1.30 (p = 0.55)]. Mortality was significantly higher in the cooled infants when compared with the control infants (42.4% versus 31.3%; p = 0.02) (Fig. 3). There was no difference in neurodisability amongst survivors, or in survival with normal neurological outcomes. Severe microcephaly was seen in 17.2% of the cooled infants and 17% of the usual care infants. Cooling was also found to increase bleeding in general with severe thrombocytopenia, gastric bleeding, and pulmonary haemorrhages. Blood stream positive sepsis was similar in both groups and was reported in less than 6% of the infants. No reduction in brain injury was seen on conventional 3 T magnetic resonance imaging (MRI), spectroscopy, or whole brain fractional anisotropy. Most infants had white matter injury suggestive of partial prolonged hypoxia rather than an acute hypoxic injury.
Center-wise mortality in the HELIX trial [5]. Note that therapeutic hypothermia was associated with increased mortality at each of these tertiary neonatal intensive care units in South Asia
The HELIX trial was powered to detect a 30 percent relative risk reduction in the primary outcome from 50% in the control arm to 35% in the intervention arm, and 10% loss to follow-up. The eventual follow-up rate was much higher and only 3.5% were lost to follow-up. Hence, these data not only provide conclusive evidence of harm with therapeutic hypothermia in LMIC, but also suggest that the origins and mechanisms of brain injury in infants with LMIC is very different to that of high-income countries [5].
There were two major criticisms about the HELIX trial design that had been raised previously. Firstly, the trial was being conducted in highly selected tertiary neonatal units in South Asia, with facilities for invasive ventilation, optimal cardiovascular support, clinical expertise, and 3 Tesla MRI by a team highly experienced in therapeutic hypothermia. Secondly, an expensive automated servo-controlled device was being used [2].
The vast majority of infants in LMIC will not have access to optimal tertiary intensive care, nor expensive cooling devices. Hence, most cooling experts and international guidelines argued for a pragmatic trial using a low-cost cooling device in non-tertiary neonatal centers, rather than evaluating cooling under optimal intensive care conditions as in the HELIX trial. They argued that wider scale up and implications of the HELIX trial would be limited, particularly in less well-resourced tertiary and secondary units in India and other LMIC, as these hospitals will not have access to servo-controlled cooling devices. While these criticisms were very valid, it was important to establish the safety and efficacy of therapeutic hypothermia under optimal conditions in LMIC before testing it in a lower resource setting.
A treatment that is unsafe under optimal conditions cannot be safe in less well-resourced settings which cannot even afford a servo-controlled cooling device. Hence, the HELIX trial results are widely generalizable to all LMIC. With the publication of the HELIX trial data, continued practice of therapeutic hypothermia in LMIC, particularly in public sector tertiary neonatal centers in South Asia, could be considered as malpractice.
Improving Neonatal Research in LMIC
Dearth of evidence-based research on all aspects of hospital care of encephalopathic infants in LMIC has been highlighted in a recent review, and experts have called for urgent research into this area [26]. Meticulously conducted randomized controlled trials are the only way to establish the effectiveness of any therapy, and often clinical trials may be lifesaving, even when the trial results are negative. For example, without the data from the HELIX trial, therapeutic hypothermia would have continued to cause harm to millions of babies in India and other LMIC. However, conducting high-quality clinical trials in LMIC is a tall order, and there are considerable ethical and logistic challenges.
Ethical Issues and Research Governance
A recent systematic review reported that consent rates for neonatal intervention trials in LMIC are significantly higher [95.6%; interquartile range (IQR) 88.2–98.9] than in high-income countries (82.7%; IQR 68.6–93.0; p < 0.0010 [27], raising concerns about research governance in low-resource settings. The method and quality of obtaining consent for participation in a trial by researchers, if there are vested commercial interests in the intervention, for example trials sponsored by pharmaceutical companies, are questionable. Furthermore, disempowered and illiterate parents are unlikely to understand the difference between a randomized controlled trial and clinical management, and may often feel obliged to consent.
In the HELIX trial, extensive training was provided to the local clinical teams on research consent. The consenting process was also video recorded and audited. The quality of obtaining consent was then quantified under three domains—Empathy, Information, and Autonomy, and was scored on a scale of 1 to 5. On quantitative analysis, the median score in all the three domains was more than 4.5, indicating a very high quality of informed consent, i.e., clinicians did provide all information about the trial in an empathic way, and parents were informed that they had the choice not to participate [28].
Subsequently, two qualitative studies were performed on the process of obtaining consent. The first study was on the nuances of conversations between the parents and healthcare professionals and the second study on a qualitative interview with a selected subgroup of parents and healthcare professionals to explore their views about process of obtaining consent. These studies revealed a very different picture. The parental consent was based on an unreserved trust in the treating physician or therapeutic misconception based on a feeling that they are getting an expensive treatment free of cost. In contrast, the clinicians showed a strong bias toward cooling therapy, as it was already in wider use in India, particularly in private healthcare settings [28]. Hence it is likely that a written informed consent has little meaning in LMIC settings, and it is essential to explore novel ways of engaging parents so that meaningful research consent can be obtained.
Credibility of the Data
An elephant in the room that is rarely discussed is the credibility of the clinical research data generated from LMIC trials. In high-income countries, randomized controlled trials are taken extremely seriously. Such clinical trials are conducted only by experienced researchers and co-ordinated by clinical trials units using centralized randomisations. The cardinal principles of allocation concealment can be easily broken in single-center randomized trials using sealed envelopes.
In LMIC, clinicians neither have dedicated time to conduct high-quality research, nor have access to experienced clinical trials units. Hence, most LMIC trials are conducted by postgraduate students with little prior research experience, and often as a part of their dissertation while undertaking full-time clinical work. Poor understanding of the research methodologies, inadequate research supervision, use of databases without audit trails, single person data management, time pressures from clinical work, proliferation of predatory journals, and ambition to get quick publications with positive results—all contribute to research fraud and generate data that may cause harm. While it is important to encourage neonatal trainees to undertake research, it is probably inappropriate to undertake a clinical trial as part of a thesis submission, without adequate clinical trials unit support.
The detection of data manipulation and fraud without access to the raw data may not be easy (Fig. 4), but could be suspected when undue claims are made on data acquisition which are not realistic. Markedly delayed publication of the trial results without specific explanation or a large number of similar trials from the same institution may suggest fraud. Any policy that restricts data sharing amongst all investigators will encourage and allow fraud to flourish, and this is particularly important in multicenter studies.
Examining raw data to detect fraud: Case-report form on a baby undergoing therapeutic hypothermia in a premier tertiary neonatal unit in India. Note that the rectal (33 0C) and abdominal temperatures (34 0C) were constant during the 20-h period, mean blood pressures were recorded hourly (38 to 42 mm of Hg). All data appear to have been entered by the same person using the same pen. The center was then investigated and it became apparent that the data were being forged. The site was then removed from participating in the HELIX trial
Ensuring Adequate Neurodevelopmental Follow-Up
Most major trials consider follow-up rates of less than 90% as inadequate, and yet, there are no neonatal trials from LMIC that have reported even 80% follow-up. While the HELIX trial obtained 96.5% follow-up for the primary outcome, this required extensive commitment and resources [5]. Most large city hospitals cater to a substantial number of migrant populations, who are particularly difficult to follow up. Hence, it is extremely important to have dedicated research nurses to keep regular contact with the families and to ensure good parent engagement. The investigators need to be prepared to travel long distances and undertake neurodevelopmental assessments at the child’s home.
Collaborative Research Between High-Income Countries and LMIC
Although collaborative research between high-income countries may substantially enhance the trial quality, credibility, and impact of LMIC trials, several concerns have been raised about such collaborations. Often the research focus tends to be dictated by the needs and visions of the high-income country researchers, rather than the local needs. Such ‘parachute research’ and ‘white savior’ approaches rarely benefit the local population nor help in local capacity building in LMIC [29].
Major international funding bodies like the National Institute of Health Research (NIHR) in the UK mandates equitable partnerships and academic capacity building in LMIC as core criteria for funding international studies [30]. NIHR also mandates that the focus of the research should be based on the needs of the local population; and effective engagement of parents as equal partners is of utmost importance. Given the considerable socioeconomic barriers and disempowerment, it is important for researchers to train and support parents so that they can provide a meaningful contribution to research [30].
Academic Capacity Strengthening in LMIC
Academic capacity building in LMIC to build up a new generation of clinician scientists who are able to understand and conduct rigorous clinical trials in neonatology are vital to improve the health of LMIC newborn infants. Many global health funding schemes supported by the National Institute of Health Research, UK provide opportunities and funding for LMIC clinicians to undertake PhD at prestigious universities in the UK. Wellcome trust (UK) and Department of Biotechnology (India) alliance also provides excellent academic training and fellowship opportunities for Indian researchers.
Summary
The HELIX trial has set a new benchmark for clinical trials in neonatal medicine in LMIC and greatly elevated the bar not just of the rigor of research, quality of clinical and imaging data and follow-up, but also on involvement of parents and community in research. Generation of high-quality research data alone are not sufficient; it is important that LMIC clinicians have the necessary skills to interpret these data and use it in their clinical practice without being influenced by industry lobbying or stakeholders with vested interests. A global south alliance on prevention of neurodisability in LMIC has been recently established to conduct high-quality research in these settings, enhance capacity building, and to develop international guidelines and benchmarks (http://neurodisabilitiesalliance.com).
References
Lee AC, Kozuki N, Blencowe H, et al. Intrapartum–related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74(Suppl 1):50–72.
Saugstad OD, Robertson NJ, Vento M. A critical review of the 2020 international liaison committee on resuscitation treatment recommendations for resuscitating the newly born infant. Acta Paediatr. 2021;110:1107–12.
Thayyil S, Costello A, Shankaran S, Robertson NJ. Therapeutic hypothermia for neonatal encephalopathy implications for neonatal units in India. Indian Pediatr. 2009;46:283–9.
Thayyil S. Cooling therapy for the management of hypoxic–ischaemic encephalopathy in middle-income countries: we can, but should we? Paediatr Int Child Health. 2019;39:231–3.
Thayyil S, Pant S, Montaldo P, S S. Hypothermia for moderate or severe neonatal encephalopathy in low and middle–income countries (HELIX): a randomised control trial in India, Sri Lanka and Bangladesh. Lancet Glob Health. 2021: (in press).
Thomas N, Abiramalatha T, Bhat V, et al. Phase Changing Material for therapeutic hypothermia in neonates with hypoxic Ischemic encephalopathy -a multi–centric study. Indian Pediatr. 2018;55:201–5.
Kali GT, Martinez-Biarge M, Van Zyl J, Smith J, Rutherford M. Therapeutic hypothermia for neonatal hypoxic–ischaemic encephalopathy had favourable outcomes at a referral hospital in a middle–income country. Acta Paediatr. 2016;105:806–15.
Kali GT, Martinez-Biarge M, Van Zyl J, Smith J, Rutherford M. Management of therapeutic hypothermia for neonatal hypoxic ischaemic encephalopathy in a tertiary centre in South Africa. Arch Dis Child Fetal Neonatal Ed. 2015;100:F519–23.
Montaldo P, Pauliah S, Lally P, Olsen L, Thayyil S. Cooling in a low resource environment: Lost in translation. Semin Fetal Neonatal Med. 2014;20:72–9.
Thayyil S, Shankaran S, Wade A, et al. Whole-body cooling in neonatal encephalopathy using phase changing material. Arch Dis Child Fetal Neonatal Ed. 2013;98:F280–1.
Robertson NJ, Nakakeeto M, Hagmann C, et al. Therapeutic hypothermia for birth asphyxia in low-resource settings: a pilot randomised controlled trial. Lancet. 2008;372:801–3.
Aker K, Stoen R, Eikenes L, et al. Therapeutic hypothermia for neonatal hypoxic–ischaemic encephalopathy in India (THIN study): a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2020;105:405–11.
Tann CJ, Nkurunziza P, Nakakeeto M, et al. Prevalence of bloodstream pathogens is higher in neonatal encephalopathy cases vs. controls using a novel panel of real–time PCR assays. PLoS One. 2014;9:e97259.
Azzopardi D, Strohm B, Linsell L, et al. Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK–analysis of national data. PLoS One. 2012;7:e38504.
Zhou Wh, Gq C, Xm S, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157:367–72.
Abate BB, Bimerew M, Gebremichael B, et al. Effects of therapeutic hypothermia on death among asphyxiated neonates with hypoxic–ischemic encephalopathy: a systematic review and meta–analysis of randomized control trials. PLoS One. 2021;16:e0247229.
Aravind I, Kumar K. How two low-cost, made-in-India innovations MiraCradle & Embrace Nest are helping save the lives of newborns.In: Economic Times. 2015.Available at:https://economictimes.indiatimes.com/news/science/how–two–low–cost–made–in–india–innovations–miracradle–embrace–nest–are–helping–save–the–lives–of–newborns/articleshow/48310144.cms?from=mdr. Accessed on 13 June 2021.
Oliveira V, Kumutha JR, E N, et al. Hypothermia for encephalopathy in low-income and middle-income countries: feasibility of whole-body cooling using a low-cost servo-controlled device. BMJ Paediatr Open. 2018;2:e000245.
Thomas N, Santhanam S, Kumar M, Kuruvilla KA, Jana AK. Hypothermia for neonatal encephalopathy in resource–poor environments. J Pediatr. 2012;160:709.
Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;2013: CD003311.
Martinello KA, Meehan C, Avdic-Belltheus A, et al. Hypothermia is not therapeutic in a neonatal piglet model of inflammation-sensitized hypoxia-ischemia. Pediatr Res. 2021. https://doi.org/10.1038/s41390-021-01584-6.
Chandrasekaran M, Swamy R, Ramji S, Shankaran S, Thayyil S. Therapeutic hypothermia for neonatal encephalopathy in Indian neonatal units: a survey of national practices. Indian Pediatr. 2017;54:969–70.
Variane GF, Cunha LM, Pinto P, et al. Therapeutic hypothermia in Brazil: a multiprofessional national survey. Am JPerinatol. 2019;36:1150–6.
Joolay Y, Harrison MC, Horn AR. Therapeutic hypothermia and hypoxic ischemic encephalopathy: opinion and practice of pediatricians in South Africa. J Perinat Med. 2012;40:447–53.
Maoulainine FMR, Elbaz M, Elfaiq S, et al. Therapeutic hypothermia in asphyxiated neonates: experience from neonatal intensive care unit of university hospital of Marrakech. Int J Pediatr. 2017;2017:3674140.
Krishnan V, Kumar V, ZA B, S S, Thayyil S. Need for more evidence in the prevention and management of perinatal asphyxia and neonatal encephalopathy in low and middle–income countries: A call for action. Semin Fetal Neonatal Med. 2021: (in press).
Patterson JK, Pant S, Jones DF, et al. Informed consent rates for neonatal randomized controlled trials in low- and lower middle-income versus high-income countries: a systematic review. PLoS One.2021;16:e0248263.
Pant S, Elias MA, Woolfall K, et al. Parental and professional perceptions of informed consent and participation in a time-critical neonatal trial: a mixed-methods study in India, Sri Lanka and Bangladesh. BMJ Glob Health. 2021;6:e005757
Bockarie M, Machingaidze S, Nyirenda T, Olesen OF, Makanga M. Parasitic and parachute research in global health. Lancet Glob Health .2018;6:e964.
Global health research.In:National Institute for Health Research. 2015. Available at:https://www.nihr.ac.uk/explore–nihr/funding–programmes/global–health.htm. Accessed on 13 June 2021.
Catherine RC, Ballambattu VB, Adhisivam B, Bharadwaj SK, Palanivel C. Effect of Therapeutic Hypothermia on the Outcome in Term Neonates with Hypoxic Ischemic Encephalopathy-A Randomized Controlled Trial. J Trop Pediatr. 2021;67. https://doi.org/10.1093/tropej/fmaa073.
Chen X, Peng W, Zhang Z, et al. [Efficacy and safety of selective brain hypothermia therapy on neonatal hypoxic-ischemic encephalopathy]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018; 30:1046–50.
Rakesh K, Vishnu Bhat B, Adhisivam B, Ajith P. Effect of therapeutic hypothermia on myocardial dysfunction in term neonates with perinatal asphyxia - a randomized controlled trial. J Matern Fetal Neonatal Med. 2018;31:2418–23.
Tanigasalam V, Bhat V, Adhisivam B, Sridhar MG. Does therapeutic hypothermia reduce acute kidney injury among term neonates with perinatal asphyxia?–a randomized controlled trial. J Matern Fetal Neonatal Med. 2016;29:2545–8.
Gane BD, Bhat V, Rao R, Nandhakumar S, Harichandrakumar KT, Adhisivam B. Effect of therapeutic hypothermia on DNA damage and neurodevelopmental outcome among term neonates with perinatal asphyxia: a randomized controlled trial. J Trop Pediatr. 2014;60:134–40.
El Shimi MS, Awad HA, Hassanein SM, et al. Single dose recombinant erythropoietin versus moderate hypothermia for neonatal hypoxic ischemic encephalopathy in low resource settings. J Matern Fetal Neonatal Med. 2014;27:1295–300.
Joy R, Pournami F, Bethou A, Bhat VB, Bobby Z. Effect of therapeutic hypothermia on oxidative stress and outcome in term neonates with perinatal asphyxia: a randomized controlled trial. J Trop Pediatr. 2013;59:17–22.
Bharadwaj SK, Bhat BV. Therapeutic hypothermia using gel packs for term neonates with hypoxic ischaemic encephalopathy in resource-limited settings: a randomized controlled trial. J Trop Pediatr. 2012;58:382–8.
Bhat MA. Re: Therapeutic hypothermia following perinatal asphyxia. Arch Dis Child Fetal Neonatal Ed. 2006;91:F464;author reply F5.
Lin ZL, Yu HM, Lin J, Chen SQ, Liang ZQ, Zhang ZY. Mild hypothermia via selective head cooling as neuroprotective therapy in term neonates with perinatal asphyxia: an experience from a single neonatal intensive care unit. J Perinatol. 2006;26:180–4.
Akisu M, Huseyinov A, Yalaz M, Cetin H, Kultursay N. Selective head cooling with hypothermia suppresses the generation of platelet-activating factor in cerebrospinal fluid of newborn infants with perinatal asphyxia. Prostaglandins Leukot Essent Fatty Acids. 2003;69:45–50.
Funding
NIHR RIGHT (Research and Innovation for Global Health Transformation) program grant.
Author information
Authors and Affiliations
Contributions
V Krishnan and V Kumar drafted the manuscript under supervision of S Shankaran and S Thayyil. S Thayyil is the guarantor for this paper.
Corresponding author
Ethics declarations
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krishnan, V., Kumar, V., Shankaran, S. et al. Rise and Fall of Therapeutic Hypothermia in Low-Resource Settings: Lessons from the HELIX Trial. Indian J Pediatr (2021). https://doi.org/10.1007/s12098-021-03861-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12098-021-03861-y