Abstract
Objective
To evaluate the effect of single oral dose of 50 mg/kg clofibrate in hyperbilirubinemia of term healthy neonates in Yazd, Iran.
Methods
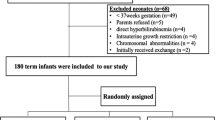
A parallel single- blinded randomized clinical trial, conducted on 60 healthy term neonates admitted between July and December 2007 to Shahid Sadoughi Hospital. Inclusion criteria were neonates with gestation age of 38–42 wk, birth weight of 2500–4000 g, product of normal vaginal delivery, breast-fed and total serum bilirubin (TSB) level of 17–29.9 mg/dL. Neonates with sepsis, anemia, severe asphyxia, hemolytic diseases, major congenital anomalies, indirect hyperbilirubinemia and underlying hepatic disorders were excluded. Selection of patients was based on random allocation via table of random numbers and the patients distributed into two groups. In group one, 30 neonates were treated with phototherapy alone and in 30 of other group treatment done with single dose of 50 mg/kg clofibrate and phototherapy. The primary endpoint with respect to efficacy in reducing of TSB was achieving TSB to less than 14 mg/dL as measured at the beginning, 12, 24 and 48 h after the start of phototherapy. Secondary outcomes were hospital stay days, duration of phototherapy and side effects of treatments during hospital stay and on the second day after discharge.
Results
No significant differences were seen from the viewpoint of rout of delivery, gender, gestational age, birth weight, hemoglobin and bilirubin level at time of admission and weight in discharge time in the two groups. After 48 h of intervention, 27 (90%) neonates in clofibrate group and 15 (56.7%) in control group had TSB of less than 14 mg/dL (p 0.02). Mean TSB 12 h after treatment (mean ± SD: 14.82 ± 1.7 mg/dL vs. 16.67 ± 1.77 mg/dL, P 0.001), 24 h after treatment (mean ± SD: 11.97 ± 2.92 mg/dL vs. 14.61 ± 2.52 mg/dL, P 0.001) and 48 h after treatment (mean ± SD: 7.91 ± 2.45 mg/dL vs. 12.74 ± 2.21 mg/dL, P 0.0001), mean of hospital stay days (mean ± SD: 1.7 ± 0.7 days vs. 3.2 ± 1.2 days, P 0.03) and duration of phototherapy (mean ± SD: 30.2 ± 13.99 h vs. 46.2 ± 15.58 h, P 0.001] were significantly lower in clofibrate group. Only loose stool was seen in two patients of clofibrate group and no significant difference was seen from view of safety of the treatments.
Conclusions
A single dose of 50 mg/kg clofibrate in treatment of neonatal hyperbilirubinemia is effective, safe and cost effective in view of reducing hospital stay days.
Similar content being viewed by others
References
Chen J, Sadakata M, Ishida M, Sekizuka N, Sayama M. Baby massage ameliorates neonatal jaundice in full-term newborn infants. Tohoku J Exp Med. 2011;223:97–102.
Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157–63.
Hankø E, Hansen TW, Almaas R, Lindstad J, Rootwelt T. Bilirubin induces apoptosis and necrosis in human NT2-N neurons. Pediatr Res. 2005;57:179.
Bhutani VK, Maisels MJ, Stark AR, Buonocore G. Expert Committee for Severe Neonatal Hyperbilirubinemia;European Society for Pediatric Research; American Academy of Pediatrics. Management of jaundice and prevention of severe neonatal hyperbilirubinemia in infants > or = 35 weeks gestation. Neonatology. 2008;94:63–7.
Cuperus FJ, Hafkamp AM, Hulzebos CV, Verkade HJ. Pharmacological therapies for unconjugated hyperbilirubinemia. Curr Pharm Des. 2009;15:2927–38.
Dennery PA. Pharmacological interventions for the treatment of neonatal jaundice. Semin Neonatol. 2002;7:111–9.
Sakha SH, Gharehbaghi MM, Rahbani ME. The effect of clofibrate with phototherapy in late pre-term newborns with non-hemolytic jaundice. Indian J Med Sci. 2009;63:174–9.
Zahedpasha Y, Ahmadpour-Kacho M, Hajiahmadi M, Naderi S, Kamali AA. Efficacy of clofibrate on severe neonatal jaundice associated with glucose-6-phosphate dehydrogenase deficiency (a randomized clinical trial). Southeast Asian J Trop Med Public Health. 2008;39:557–61.
Eghbalian F, Pourhossein A, Zandevakili H. Effect of clofibrate in non-hemolytic indirect hyperbiliru-binemia in full term neonates. Indian J Pediatr. 2007;74:1003–6.
Zahedpasha Y, Ahmadpour-Kacho M, Hajiahmadi M, Naderi S. Effect of clofibrate in jaundiced full-term infants: a randomized clinical trial. Arch Iran Med. 2007;10:349–53.
Mohammadzadeh A, Farhat ASh, Iranpour R. Effect of clofibrate in jaundiced term newborns. Indian J Pediatr. 2005;72:123–6.
Badeli HR, Sharafi R, Sajedi SA. The effect of clofibrate on neonatal hyperbilirubinemia in uncomplicated jaundice. Iran J Ped. 2008;18:20–4.
Caballero Noguez B, Hernadez PS, Rodriguez JBE, et al. Clofibrate effect associated with phototherapy on bilirubin concentration in newly-born babies. Rev Mex Pediatr. 2001;68:176–80.
Lindenbaum A, Delaporte B, Benattar C, Dehan M, Magny JF, Gerbet D, et al. Preventive treatment of jaundice in premature newborn infants with clofibrate. Double-blind controlled therapeutic trial. Arch Fr Pediatr. 1985;42:759–63.
Lindenbaum A, Hernandorena X, Vial M, et al. Clofibrate for the treatment of hyperbilirubinemia in neonates born at term: a double blind controlled study. Arch Fr Pediatr. 1981;38:867–73. Article in French.
Bourget P, Broise I, Quinquis-Desmaris V, Gabilan JC. Pharmacokinetics of clofibrate in jaundiced newborn infants at term. Arch Pediatr. 1995;2:722–8. Article in French.
Huang MJ, Kua KE, Teng KS, Weng HW, Huang CS. Risk factors for severe hyperbilirubinemia in neonates. Pediatr Res. 2004;56:677–8.
Moslehi MA, Pishva N. Determination of effect of low dose vs moderate dose clofibrate on decreasing serum bilirubin in healthy term neonates. Iran J Ped. 2007;17:108–12.
Wazir S, Angiti RR, Kumar P. Effect of clofibrate in jaundiced term neonates. Indian J Pediatr. 2006;73:170.
Gabilan JC. Pharmacologic treatment of neonatal jaundice. A new approach. Arch Pediatr. 1998;5:1274–8.
Conflict of Interest
None.
Role of Funding Source
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fallah, R., Islami, Z. & Lotfi, S.R. Single Dose of 50 mg/kg Clofibrate in Jaundice of Healthy Term Neonates: Randomised Clinical Trial of Efficacy and Safety. Indian J Pediatr 79, 194–197 (2012). https://doi.org/10.1007/s12098-011-0531-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-011-0531-9