Abstract
Purpose
Despite known high-risk features, accurate identification of patients at high risk of cancer recurrence in colon cancer remains a challenge. As tumour stroma plays an important role in tumour invasion and metastasis, the easy, low-cost and highly reproducible tumour-stroma ratio (TSR) could be a valuable prognostic marker, which is also believed to predict chemo resistance.
Methods
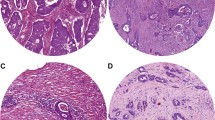
Two independent series of patients with colon cancer were selected. TSR was estimated by microscopic analysis of 4 µm haematoxylin and eosin (H&E) stained tissue sections of the primary tumour and the corresponding metastatic lymph nodes. Patients were categorized as TSR-low (≤ 50%) or TSR-high (> 50%). Differences in overall survival and cancer-free survival were analysed by Kaplan–Meier curves and cox-regression analyses. Analyses were conducted for TNM-stage I–II, TNM-stage III and patients with an indication for chemotherapy separately.
Results
We found that high TSR was associated with poor cancer-free survival in TNM-stage I–II colon cancer in two independent series, independent of other known high-risk features. This association was also found in TNM-stage III tumours, with an additional prognostic value of TSR in lymph node metastasis to TSR in the primary tumour alone. In addition, high TSR was found to predict chemo resistance in patients receiving adjuvant chemotherapy after surgical resection of a TNM-stage II–III colon tumour.
Conclusion
In colon cancer, the TSR of both primary tumour and lymph node metastasis adds significant prognostic value to current pathologic and clinical features used for the identification of patients at high risk of cancer recurrence, and also predicts chemo resistance.


Similar content being viewed by others
Data availability
Data and material cannot be shared publicly because of ethical concerns. Patients were included on a no objection base to conduct retrospective data studies and publish findings, but were not asked for permission to publish full encrypted data. Data are available from the VieCuri Institutional Data Access (contact via wetenschapsbureau@viecuri.nl) for researchers who meet the criteria for access to confidential data.
Code availability
Codes are available upon request via corresponding author.
References
Dutch guideline 'colonic cancer' 2019. https://richtlijnendatabase.nl/richtlijn/colorectaal_carcinoom_crc/primaire_behandeling_coloncarcinoom_bij_crc.html.
van Pelt GW, Sandberg TP, Morreau H, Gelderblom H, van Krieken J, Tollenaar R, et al. The tumour-stroma ratio in colon cancer: the biological role and its prognostic impact. Histopathology. 2018;73(2):197–206.
Hyslop T, Waldman SA. Molecular staging of node negative patients with colorectal cancer. J Cancer. 2013;4(3):193–9.
O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96(19):1420–5.
Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22(16):3408–19.
O’Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29(25):3381–8.
Conti J, Thomas G. The role of tumour stroma in colorectal cancer invasion and metastasis. Cancers (Basel). 2011;3(2):2160–8.
De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123(10):2229–38.
Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22(5):571–84.
Hawinkels LJ, Paauwe M, Verspaget HW, Wiercinska E, van der Zon JM, van der Ploeg K, et al. Interaction with colon cancer cells hyperactivates TGF-beta signaling in cancer-associated fibroblasts. Oncogene. 2014;33(1):97–107.
Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3(11):807–21.
Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12(5):468–76.
Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60.
Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330(6005):827–30.
Sandberg TP, Stuart M, Oosting J, Tollenaar R, Sier CFM, Mesker WE. Increased expression of cancer-associated fibroblast markers at the invasive front and its association with tumor-stroma ratio in colorectal cancer. BMC Cancer. 2019;19(1):284.
Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274(51):36505–12.
Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8(9):705–13.
Hale MD, Hayden JD, Grabsch HI. Tumour-microenvironment interactions: role of tumour stroma and proteins produced by cancer-associated fibroblasts in chemotherapy response. Cell Oncol (Dordr). 2013;36(2):95–112.
Mesker WE, Junggeburt JM, Szuhai K, de Heer P, Morreau H, Tanke HJ, et al. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol. 2007;29(5):387–98.
Park JH, Richards CH, McMillan DC, Horgan PG, Roxburgh CSD. The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Ann Oncol. 2014;25(3):644–51.
Huijbers A, Tollenaar RA, v Pelt GW, Zeestraten EC, Dutton S, McConkey CC, et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: validation in the VICTOR trial. Ann Oncol. 2013;24(1):179–85.
van Pelt GW, Hansen TF, Bastiaannet E, Kjaer-Frifeldt S, van Krieken JHJ, Tollenaar RA, et al. Stroma-high lymph node involvement predicts poor survival more accurately for patients with stage III colon cancer. J Med Surg Pathol. 2016;1:1–8.
Vangangelt KMH, Green AR, Heemskerk IMF, Cohen D, van Pelt GW, Sobral-Leite M, et al. The prognostic value of the tumor-stroma ratio is most discriminative in patients with grade III or triple-negative breast cancer. Int J Cancer. 2020;146(8):2296–304.
van Pelt GW, Kjaer-Frifeldt S, van Krieken J, Al Dieri R, Morreau H, Tollenaar R, et al. Scoring the tumor-stroma ratio in colon cancer: procedure and recommendations. Virchows Arch. 2018;473(4):405–12.
Smit MA, van Pelt GW, Dequeker EM, Al Dieri R, Tollenaar RA, van Krieken JHJ, et al. e-Learning for instruction and to improve reproducibility of scoring tumor-stroma ratio in colon carcinoma: performance and reproducibility assessment in the UNITED study. JMIR Form Res. 2021;5(3):e19408.
Eriksen AC, Sorensen FB, Lindebjerg J, Hager H, de Pont Christensen R, Kjaer-Frifeldt S, et al. The prognostic value of tumour stroma ratio and tumour budding in stage II colon cancer. A nationwide population-based study. Int J Colorectal Dis. 2018;33(8):1115–24.
Hutchins GGA, Treanor D, Wright A, Handley K, Magill L, Tinkler-Hundal E, et al. Intratumoral stromal morphometry predicts disease recurrence but not response to 5-fluorouracil-results from the QUASAR trial of colorectal cancer. Histopathology. 2018;72(3):391–404.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
McKay JA, Douglas JJ, Ross VG, Curran S, Ahmed FY, Loane JF, et al. Expression of cell cycle control proteins in primary colorectal tumors does not always predict expression in lymph node metastases. Clin Cancer Res. 2000;6(3):1113–8.
McKay JA, Murray LJ, Curran S, Ross VG, Clark C, Murray GI, et al. Evaluation of the epidermal growth factor receptor (EGFR) in colorectal tumours and lymph node metastases. Eur J Cancer. 2002;38(17):2258–64.
Zalata KR, Elshal MF, Foda AA, Shoma A. Genetic dissimilarity between primary colorectal carcinomas and their lymph node metastases: ploidy, p53, bcl-2, and c-myc expression—a pilot study. Tumour Biol. 2015;36(8):6579–84.
Hagenaars SC, de Groot S, Cohen D, Dekker TJA, Charehbili A, Meershoek-Klein Kranenbarg E, et al. Tumor-stroma ratio is associated with Miller-Payne score and pathological response to neoadjuvant chemotherapy in HER2-negative early breast cancer. Int J Cancer. 2021.
van Pelt GW, Krol JA, Lips IM, Peters FP, van Klaveren D, Boonstra JJ, et al. The value of tumor-stroma ratio as predictor of pathologic response after neoadjuvant chemoradiotherapy in esophageal cancer. Clin Transl Radiat Oncol. 2020;20:39–44.
Lotti F, Jarrar AM, Pai RK, Hitomi M, Lathia J, Mace A, et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J Exp Med. 2013;210(13):2851–72.
Yeldag G, Rice A, Del Rio Hernandez A. Chemoresistance and the self-maintaining tumor microenvironment. Cancers (Basel). 2018;10(12).
Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62(12):3387–94.
Lee KA, Roth RA, LaPres JJ. Hypoxia, drug therapy and toxicity. Pharmacol Ther. 2007;113(2):229–46.
Sriraman SK, Aryasomayajula B, Torchilin VP. Barriers to drug delivery in solid tumors. Tissue Barriers. 2014;2:e29528.
Zhao J. Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacol Ther. 2016;160:145–58.
Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15(6):366–81.
UNITED Study. http://watchstroma.com.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Conceptualization: MTAS, FJV. Data curation: MTAS, TKEF, AHLMG, RLAvdL, FJV. Formal analysis: MTAS. Investigation: MTAS, TKEF, AHLMG, APdB. Methodology: MTAS, MLGJ-H. Project administration: MTAS, FJV. Resources: MTAS, RLAvdL, WEM, KB, CMB, FJV, APdB. Supervision: MLGJ-H, FJV, APdB. Validation: MTAS, RLAvdL, WEM. Visualization: MTAS. Writing—original draft: MTAS. Writing—review and editing: TKEF, RLAvdL, WEM, KB, CMB, MLGJ-H, FJV, APdB.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interest exists.
Ethical approval (Research involving human participants and/or animals)
This study was approved by the research committee and the Board of Directors of VieCuri Medical Centre and Jeroen Bosch Hospital. Data were obtained under the law ‘scientific research and statistics in the interest of public health, where asking for permission is not possible or appropriate for several reasons’ in the Netherlands, unless patients objected to use of their personal medical record for scientific research. Data were encrypted with an encryption key provided by the NCR. Encryption was shortly lifted to access the patients’ number for accessing his/her medical record. Following extraction data were encrypted again. Prior collected material was reassessed. A waiver of informed consent was given by the METC Maastricht under the reference number 2020-1336.
Informed consent
Data were obtained under the law ‘scientific research and statistics in the interest of public health, where asking for permission is not possible or appropriate for several reasons’ in the Netherlands, unless patients objected to use of their personal medical record for scientific research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Strous, M.T.A., Faes, T.K.E., Gubbels, A.L.H.M. et al. A high tumour-stroma ratio (TSR) in colon tumours and its metastatic lymph nodes predicts poor cancer-free survival and chemo resistance. Clin Transl Oncol 24, 1047–1058 (2022). https://doi.org/10.1007/s12094-021-02746-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02746-y