Abstract
Purpose
Immune cells such as cytotoxic T cells, helper T cells, B cells or tumor-associated macrophages (TAMs) contribute to the anti-tumor response or pro-tumorigenic effect in triple negative breast cancer (TNBC). The interrelation of TAMs, T and B tumor-infiltrating lymphocytes (TILs) in TNBC has not been fully elucidated.
Methods
We evaluated the association of tumor-associated macrophages, T and B TILs in TNBC.
Results
TNBCs with a high CD68+, CD163+ TAMs and low CD4+, CD8+, CD20+ TILs had a significantly shorter relapse-free survival (RFS) and overall survival (OS) than those with low CD68+, CD163+ TAMs and high CD4+, CD8+, CD20+ TILs. TNBCs with high CD68+ TAMs/low CD8+ TILs showed a significantly shorter RFS and OS and a significantly poorer prognosis than those with high CD68+ TAMs/high CD8+ TILs, low CD68+ TAMs/high CD8+ TILs, and low CD68+/low CD8+. TNBCs with high CD163+ TAMs/low CD8+, low CD20 + TILs showed a significantly shorter RFS and OS and a significantly poorer prognosis than those with high CD163+ TAMs/high CD8+ TILs and high CD163+ TAMs /high CD20+ TILs.
Conclusions
Our study suggests that TAMs further create an optimal tumor microenvironment (TME) for growth and invasion of cancer cells when evasion of immunoreactions due to T and B TILs occurs. In TNBCs, all these events combine to affect prognosis. The process of TME is highly complex in TNBCs and for an improved understanding, larger validation studies are necessary to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancers historically described as medullary carcinoma, or carcinoma with medullary features, were previously recognized as a specific, special type of well-circumscribed breast cancer with a prominent tumor-infiltrating lymphocyte (TIL) and macrophage infiltrate, and associated with better prognosis than other stage-matched high-grade cancers. However, “carcinoma with medullary features” has suffered from poor interobserver reproducibility and overlapping features with Triple-negative breast cancer (TNBC). TNBC is characterized by a lack of expression of the estrogen receptor (ER) and progesterone receptor (PgR), and absence of human epidermal growth factor receptor 2 (HER2) protein overexpression; this type is known to have a poor prognosis and to recruit TILs and tumor-associated macrophages (TAMs). Usually, hormone therapy and drugs that target HER2 are not helpful in TNBC, so chemotherapy is the main systemic treatment option. In addition, there is currently no consideration of these immune cells in the therapeutic approach. A more recent discovery of the prognostic importance of TILs in high-grade breast cancers appears to explain the good prognosis of these cancers [1,2,3]. The latest WHO classification proposed considering carcinomas with a medullary pattern as representing one end of the spectrum of TIL-rich invasive breast carcinoma of no special type (IBC-NSTs), rather than a distinct morphological subtype, and to use the term “IBC-NST with medullary pattern” [4].
According to these series of theories, we previously reported that CD20+ TILs may support an increase in CD4+ and CD8+ TILs, altering the anti-tumor response and resulting in a positive prognosis in TNBC [5,6,7]. Further, although the prognostic correlation with macrophages has been widely reported in breast cancer, there is no consensus on the prognostic impact [8,9,10,11,12]. TAMs have recently been reported as an important factor in tumor growth and cancer progression. Recently, two processes have been proposed for TAMs activation: Classically-activated type 1 (M1-like) macrophages and alternatively-activated type 2 (M2-like) macrophages. M1-like macrophages, characterized by CD68 expression, produce free radicals that can lead to DNA damage with the potential to contribute to tumoricidal activity [13]. In contrast, M2-like macrophages, characterized by both CD68 and CD163 expression, are considered to promote tumor growth and metastasis by releasing chemokines, which are inflammatory growth factors [14, 15].
CD68+ and CD163+ are the most commonly reported when identifying TAM. In addition, we previously reported that infiltration of CD163+ TAMs, rather than CD68+, was associated with poor prognosis in TNBC patients by multivariate analysis [16]. To date, only a few studies have investigated TILs and macrophages in combination [17,18,19]. Recent discoveries about the immune system have drastically changed conventional assumptions regarding the role of macrophages and lymphocytes in anti-tumor activity [6, 20,21,22,23]. TAMs cooperate with T and B TILs based on the release of chemokines, cytokines with reactive radicals among other proteins. Recently, tumor microenvironment, such as TAMs and TILs have been considered important prognostic factors in cancer. However, the interrelation of TAMs, T and B TILs in TNBC has not been fully elucidated. The purpose of the present study was to evaluate the CD68+ and CD163+ TAMs, CD4+, CD8+ T TILs and CD20+ B TILs in TNBC and examine their clinicopathological features and correlations.
Methods
Patients and tissue specimens
A total 107 cases of TNBC who had operations from 2006 to 2018 in Dokkyo Medical University hospital were used in the present study. The explored clinicopathological parameters included age, tumor size, histologic grade, histology, lymph node status, mib-1 index, unstained TILs, and follow-up data. This study was conducted according to the Declaration of Helsinki and after approval by the ethics committee of Dokkyo Medical University (No.28009).
Immunohistochemistry (IHC) was performed on formalin-fixed and paraffin-embedded sections using an antibody to ER (clone SP1, Novocastra (Leica), prediluted, nuclear), PgR (clone 1E2, Novocastra (Leica), prediluted, nuclear), HER2 (clone 4B5, Roche (VENTANA), prediluted, membranous), CD4 (CD4, clone 1F6, Novocastra (Leica), 1:40), CD8 (CD8, clone 4B11, Novocastra (Leica), prediluted), CD20 (CD20, clone L26, Nichirei), CD68 (CD68, clone PG-M1, Dako (Agilent), 1:50), and CD163 (CD163, clone 10D6, Novocastra (Leica), 1:50), ki67 (mib-1, clone K-2, Novocastra (Leica)) according to the manufacturer’s instructions. For every IHC staining, tonsil specimens were used as positive and negative controls.
The percentages of nuclei stained for ER and PgR expression were analyzed using the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines of a threshold of 1% [24]. HER2 expression was assessed according to the guidelines determined by ASCO/CAP [25]. Analysis of unstained TILs was performed on hematoxylin and eosin-stained sections according to the criteria proposed by the International Immuno-Oncology Biomarkers Working Group [26]. Unstained TILs were defined as lymphocytes located within the stroma and stratified as high (≥ 30%) and low (< 30%) [27]. The mib-1 index was derived from the sum total of the percentages of different staining intensities, and threshold of the set at 20% [28].
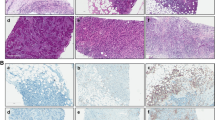
The density of tumor-infiltrating immune cell subsets in the tumor stroma of TNBC was quantified as total counts of CD4, CD8, CD20, CD68, and CD163-positive cells per high power field by manual inspection of stained sections with five areas of high staining intensity. Each specimen was screened at low magnification (×100), and the greatest number of positively stained cells (hot spot area) was selected for the subsequent analysis. The mean tumor-infiltrating immune cell counts in these areas for each case were evaluated. Infiltrating immune cells, as identified by the different markers, and the number of positive cells were divided into lower and higher groups (Fig. 1) based on cut-off points according to the median. As a result, the cut-off for CD4 was 104, CD8 was 81, CD20 was 60, CD68 was 26.2, and CD163 was 26.6. All slides were estimated by two pathologists (HK and TJ) who had no access to the clinical data.
Statistical analysis
All statistical analysis was performed using SPSS 26.0 (IBM Corporation, Armonk, NY, USA). A p value of < 0.05 was regarded as significant and all statistical tests were two-sided. Correlation analyses between TAMs or TILs and clinicopathological parameters were done using Pearson’s chi-square test. The correlation of TAMs and TILs with relapse-free survival (RFS) and overall survival (OS) was analyzed by Kaplan–Meier analysis. Significance was evaluated using the log-rank test. Cox proportional hazard models were used to estimate hazard ratios for death from breast cancer according to the correlation of TAMs and TILs in both univariate and multivariate analyses.
Results
The clinicopathological parameters of the patients are summarized in Tables 1 and 2. The density of CD68+ TAMs/CD8+ TILs was significantly associated with histology (p = 0.020) and the mib-1 index (p = 0.043). Moreover, the densities of CD68+ TAMs/CD8+ TILs and CD68+ TAMs/CD20+ TILs were significantly related to expression of unstained TILs (p = 0.022, p < 0.001). Additionally, histological grade was significantly associated with CD163+ TAMs/CD4+ TILs or CD8+ TILs or CD20+ TILs (p < 0.001, p < 0.001, and p = 0.001; respectively). The CD163+ TAMs/CD4+ TILs (p = 0.038) and CD163+ TAMs/CD8+ TILs (p = 0.020) were correlated with histology. Furthermore, CD163+ TAMs/CD8+ TILs and CD163+ TAMs/CD20+ TILs were significantly related to expression of unstained TILs (p = 0.022, p < 0.001). However, there was no significant difference found in other clinicopathological parameters.
RFS and OS rates for all groups are shown by Kaplan–Meier curves and differences were analyzed by the log-rank test (Figs. 2, 3). Patients with a high CD68+ TAMs/low CD4+ TILs phenotype had a statistically significant shorter RFS and OS compared with patients with high CD68+ TAMs/high CD4+ TILs (RFS: p = 0.040) and low CD68+ TAMs/high CD4+ TILs (RFS: p = 0.019, OS: p = 0.019). There was no significant difference found among high CD68+ TAMs/low CD4+ TILs, high CD68+ TAMs/high CD4+ TILs and low CD68+ TAMs/low CD4+ TILs (RFS: p = 0.436, OS: p = 0.296). Patients with a high CD68+ TAMs/low CD8+ TILs phenotype had a statistically significant shorter RFS and OS compared with those with high CD68+ /high CD8+ TILs (RFS: p = 0.020, OS: p = 0.023), low CD68+ TAMs/high CD8+ TILs (RFS: p = 0.001, OS: p = 0.001), and low CD68+ TAMs/low CD8+ TILs (RFS: p = 0.014, OS: p = 0.014). Patients with a high CD68+ TAMs/low CD20+ TILs phenotype had a statistically significant shorter RFS and OS compared with patients with low CD68+ TAMs/high CD20+ TILs (RFS: p = 0.008, OS: p = 0.003). No significant difference was found among high CD68+ TAMs/low CD20+ TILs, high CD68+ TAMs/high CD20+ TILs (RFS: p = 0.210, OS: p = 0.147) and low CD68+ TAMs/low CD20+ TILs (RFS: p = 0.839, OS: p = 0.983).
Kaplan–Meier curves showing the relapse-free survival and overall survival of patients with certain densities of CD68+ TAMs versus densities of TILs. a, b CD68+ TAMs/CD4+ TILs. c, d CD68+ TAMs/CD8+ TILs. e, f CD68+ TAMs/CD20+ TILs. TILs tumor-infiltrating lymphocytes, TAMs tumor-associated macrophages
Kaplan–Meier curves showing the relapse-free survival and overall survival of patients with certain densities of CD163+ TAMs versus densities of TILs. a, b CD163+ TAMs/CD4+ TILs. c, d CD163+ TAMs/CD8+ TILs. e, f CD163+ TAMs/CD20+ TILs. TILs tumor-infiltrating lymphocytes, TAMs tumor-associated macrophages
For TAM2s/TILs, patients with a high CD163+ TAMs/low CD4+ TILs phenotype had a statistically significant shorter RFS and OS compared with those with high CD163+ TAMs/high CD4+ TILs (RFS: p = 0.033), low CD163+ TAMs/high CD4+ TILs (RFS: p = 0.001, OS: p = 0.002) and low CD163+ TAMs/low CD4+ TILs (RFS: p = 0.025, OS: p = 0.034). No significant difference was observed between high CD163+ TAMs/lowCD4+ TILs and high CD163+ TAMs/high CD4+ TILs (OS: p = 0.061) in OS. Patients with a high CD163+ TAMs/low CD8+ TILs phenotype had a statistically significant shorter RFS and OS compared with those with high CD163+ TAMs/high CD8+ TILs (RFS: p = 0.020, OS: p = 0.023), low CD163+ TAMs/high CD8+ TILs (RFS: p = 0.001, OS: p = 0.001) and low CD163+ TAMs/low CD8+ TILs (RFS: p = 0.014, OS: p = 0.014). Patients with a high CD163+ TAMs/low CD20+ TILs phenotype had a statistically significant shorter RFS and OS compared with those with high CD163+ TAMs/high CD20+ TILs (RFS: p = 0.037, OS: p = 0.019), low CD163+ TAMs/high CD20+ TILs (RFS: p < 0.001, OS: p < 0.001) and low CD163+ TAMs/low CD20+ TILs (OS: p = 0.047). There was no significant difference between high CD163+ TAMs/low CD20+ TILs and low CD163+ TAMs/low CD20+ TILs (RFS: p = 0.053) in RFS.
To evaluate the prognostic implications of CD68+ and CD163+ TAMs in relation to infiltrating CD4+, CD8+, CD20+ TILs, we compared the prognostic value of groups of CD68+ or CD163+ (high or low) to those of CD4+, CD8+ and, CD20+ (high or low) (Table 3 and 4). In the multivariate assessment, patients with a high CD68+ TAMs/low CD8+ TILs phenotype had a statistically significantly poorer prognosis compared to patients with high CD68+ TAMs/high CD8+ TILs (RFS: hazard ratio (HR) 0.334, 95% CI 0.117–0.952, p = 0.040, OS: HR 0.330, 95% CI 0.116–0.940, p = 0.038), low CD68+ TAMs/high CD8+ TILs (RFS: HR 0.073, 95% CI 0.009–0.569, p = 0.013, OS: HR 0.074, 95% CI 0.009–0.576, p = 0.013), and low CD68+ TAMs/low CD8+ TILs (RFS: HR 0.214, 95% CI 0.055–0.827, p = 0.025, OS: HR 0.219, 95% CI 0.056–0.861, p = 0.030).
Patients with a high CD163+ TAMs/low CD8+ TILs phenotype had a statistically significant poorer prognosis compared with patients with high CD163+ TAMs/high CD8+ TILs (RFS: HR 0.339, 95% CI 0.118–0.969, p = 0.044, OS: HR 0.344, 95% CI 0.121–0.984, p = 0.047). Interestingly, patients with a high CD163+ TAMs/low CD8+ TILs phenotype had a statistically significant shorter OS compared to patients with CD163+ TAMs/high CD8+ TILs (OS: HR 0.336, 95% CI 0.117–0.915, p = 0.043). However, no significant difference was observed in RFS between high CD163+ TAMs/low CD8+ TILs and high CD163+ TAMs/high CD8+ TILs (RFS: HR 0.378, 95% CI 0.132–1.086, p = 0.071).
Discussion
Our study revealed that high TAMs (CD68, CD163) with low TILs (CD4, CD8, CD20) correlated significantly with poor prognosis in TNBCs. Further, multivariate analysis also showed that CD68/CD8, CD163/CD8, and CD163/CD20 were associated with prognosis.
The first explanation for this result is that immune evasion caused by TAM. TAMs usually play an important role in the human immune system against tumors, and they can induce specific immunity by promoting activation and recruitment of T and B TILs (Fig. 4). They are important to elicit an appropriate immune response [13,14,15]. B cells T lymphocytes play an essential part in immune defense. Traditionally, these cells are divided into two subtypes, CD4+ T helper cells and CD8+ cytotoxic T cells [29]. The first subtype can help B TILs to induce antibodies to produce immune activity through the recruitment of various immune cells to appropriate sites associated with macrophage response and inflammation. The second type is essential for protection against cytopathogens, among other functions. These immune reactions contribute to pathogen protection, inflammation mitigation, and antibody production. Despite previous reports that suggested a favorable prognosis with early T TILs in TNBCs, successful tumors that progress and become lethal to patients are clearly not eliminated. This immune evasion is at least partly blocked by TAMs, but also involves regulatory T cells, as well as tumor cell immune evasion [30,31,32]. CD4+ T TILs recruitment in tumors often follows a regulatory T cell (Treg) subset, which leads to severe immunodeficiency and can be produced by TAMs through cytokine expression, as reported in ovarian cancer patients [33]. Similarly, CD4+ T TILs were shown to differentiate into Tregs when co-cultured with TAMs from glioblastoma patients [34]. However, the functional role CD4+ T TILs play in immunological response is complex [35]. CD4+ T TILs with cytotoxic capacity were shown to reject tumor cells and incorporate B TILs release of cytokines to drive cytotoxic immune responses to the tumor cells. The increased expression of CD8+ T TILs was also partly regulated by TAMs and was more pronounced at killing tumors than CD4+ T TILs, for which Treg was more prominent [36, 37].
Schema of the interaction of immune cells in the breast cancer tumor microenvironment. CD4+ /CD8+ /CD20+ TILs directly kill the tumor cells. TILs are supported by TAM1s and suppress the tumor cells. In contrast, TAM2s exerts an immunosuppressive function via the inhibition of T cells and B cells, indicating tumorigenic roles. TILs tumor-infiltrating lymphocytes, TAM1s tumor-associated macrophages-1, TAM2s tumor-associated macrophages-2
In this important context, the combination of a high density of TAMs and low infiltration of CD4+, CD8+ T TILs in human breast cancer is indicative of poorer survival [38]. In our study, TNBCs with absent/low infiltration of T and B TILs and a high density of CD68+, CD163+ TAMs had a statistically significant shorter RFS and OS. Similar to our findings, several articles reported that a high density of TAMs was associated with poor prognosis in patients with prostate, urinary bladder, kidney and breast cancer [12, 39,40,41].
The second explanation is that TAMs can contribute to tumor destruction and influence tumor growth and progression themselves. Previously, we also reported CD163+ TAM2 are correlated in TNBC. TAM receptors are reported to be overexpressed in both solid and hematological malignancies, and high expression of the TAM receptors has been associated with poor patient survival in a variety of cancers. Oncogenic TAM receptor signaling results in increased proliferation, cell survival and metastasis. Actually, our report suggests that CD163+ TAMs may correlate with the poor prognosis of TNBC [16]. However, in this study, even if CD163+ TAM was expressed, the prognosis differed depending on the degree of TIL infiltration. This may be because CD163+ TAM promotes cancer infiltration, while TIL causes an immune response to the cancer, affecting prognosis. Similar results of TAM were obtained with CD68, but were not as significant as with CD163. Some studies of CD68+ TAM involving patients with melanoma, gastric and colorectal cancer reported contrasting findings [42,43,44].
A possible inference from these opposing results may be the use of a pan-macrophage marker for TAMs. CD68 determines not only TAM2, but also TAM1, which generates free radicals that can induce DNA damage with the potential for tumoricidal activity. Thus, our study suggests that CD163+ TAMs further create an optimal TME for growth and invasion of cancer cells when evasion of immunoreactions by T and B TILs occurs. However, CD68+ TAMs that are thought to be partly derived from TAM1s, when with CD8+ TILs, are suggested to be involved in effective anti-tumor immunity.
Conclusion
Our study revealed that high TAMs (CD68, CD163) with low TILs (CD4, CD8, CD20) correlated significantly with poor prognosis in TNBCs. Further, multivariate analysis also showed that CD68/CD8, CD163/CD8, and CD163/CD20 were associated with prognosis. Our study suggests that CD163+ TAMs further create an optimal TME for growth and invasion of cancer cells when evasion of immunoreactions by T and B TILs occurs. CD68+ TAMs, which are thought to be partly derived from TAM1s are thought to be involved in effective human anti-tumor immunity. In TNBCs, all these events combine to affect prognosis. The process of TME is highly complex in TNBCs and for an improved understanding, larger validation studies are necessary to confirm these findings.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Park JH, Jonas SF, Bataillon G, Criscitiello C, Salgado R, Loi S, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol. 2019;30:1941–9.
Matkowski R, Gisterek I, Halon A, Lacko A, Szewczyk K, Staszek U, et al. The prognostic role of tumor-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Anticancer Res. 2009;29:2445–51.
Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25:1536–43.
Lokuhetty D, White V, Watanabe R, Cree I. WHO classification of breast tumours, vol. 2. 5th ed. Lyon: IARC; 2019.
Jamiyan T, Kuroda H, Yamaguchi R, Nakazato Y, Noda S, Onozaki M, et al. Prognostic impact of a tumor-infiltrating lymphocyte subtype in triple negative cancer of the breast. Breast Cancer. 2020;27:880–92.
Kuroda H, Jamiyan T, Yamaguchi R, Kakumoto A, Abe A, Harada O, et al. Tumor-infiltrating B cells and T cells correlate with postoperative prognosis in triple-negative carcinoma of the breast. BMC Cancer. 2021;21:286.
Kuroda H, Jamiyan T, Yamaguchi R, Kakumoto A, Abe A, Harada O, et al. Prognostic value of tumor-infiltrating B lymphocytes and plasma cells in triple-negative breast cancer. BMC Cancer. 2021;21:286.
Jeong H, Hwang I, Kang SH, Shin HC, Kwon SY. Tumor-associated macrophages as potential prognostic biomarkers of invasive breast cancer. J Breast Cancer. 2019;22:38–51.
Shabo I, Stal O, Olsson H, Doré S, Svanvik J. Breast cancer expression of CD163, a macrophage scavenger receptor, is related to early distant recurrence and reduced patient survival. Int J Cancer. 2008;123:780–6.
Tsutsui S, Yasuda K, Suzuki K, Tahara K, Higashi H, Era S. Macrophage infiltration and its prognostic implications in breast cancer: the relationship with VEGF expression and microvessel density. Oncol Rep. 2005;14:425–31.
Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–9.
Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65:159–63.
Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol. 2011;89:557–63.
Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73.
Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35.
Jamiyan T, Kuroda H, Yamaguchi R, Abe A, Hayashi M. CD68- and CD163-positive tumor-associated macrophages in triple negative cancer of the breast. Virchows Arch. 2020;477:767–75.
DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212.
Miura T, Yoshizawa T, Hirai H, Seino H, Morohashi S, Wu Y, et al. Prognostic impact of CD163+ macrophages in tumor stroma and CD8+ T-cells in cancer cell nests in invasive extrahepatic bile duct cancer. Anticancer Res. 2017;37:183–90.
Zhu Y, Li M, Bo C, Liu X, Zhang J, Li Z, et al. Prognostic significance of the lymphocyte-to-monocyte ratio and the tumor-infiltrating lymphocyte to tumor-associated macrophage ratio in patients with stage T3N0M0 esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:343–54.
Nielsen JS, Sahota RA, Milne K, Nielsen J, Sahota R, Milne K, Kost S, Nesslinger N, Watson P, et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281–92.
Garnelo M, Tan A, Her Z, Yeong J, Lim C, Chen J, et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66:342–51.
Shi JY, Gao Q, Wang ZC, Zhou J, Wang X, Min Z, et al. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res. 2013;19:5994–6005.
Edin S, Kaprio T, Hagstrom J, Larsson P, Mustonen H, Böckelman C, et al. The prognostic importance of CD20(+) B lymphocytes in colorectal cancer and the relation to other immune cell subsets. Sci Rep. 2019;9:19997–8.
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38:1346–66.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018;142:1364–82.
Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the international immunooncology biomarkers working group: part 1: assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017;24:235–51.
Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37:559–69.
Tashima R, Nishimura R, Osako T, Nishiyama Y, Okumura Y, Nakano M, et al. Evaluation of an optimal cut-off point for the Ki-67 index as a prognostic factor in primary breast cancer: a retrospective study. PLoS ONE. 2015;10:e0119565.
Mitchison NA. T-cell-B-cell cooperation. Nat Rev Immunol. 2004;4:308–12.
Coussens LM, Pollard JW. Leukocytes in mammary development and cancer. Cold Spring Harb Perspect Biol. 2011;3:a003285.
Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, Spranger S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr Opin Immunol. 2013;25:268–76.
Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39.
Zhu Q, Wu X, Wu Y, Wang X. Interaction between Treg cells and tumor-associated macrophages in the tumor microenvironment of epithelial ovarian cancer. Oncol Rep. 2016;36:3472–8.
Li Z, Liu X, Guo R, Wang P. CD4(+) Foxp3(−) type 1 regulatory T cells in glioblastoma multiforme suppress T cell responses through multiple pathways and are regulated by tumor-associated macrophages. Int J Biochem Cell Biol. 2016;81:1–9.
Ahrends T, Borst J. The opposing roles of CD4(+) T cells in anti-tumour immunity. Immunology. 2018;154:582–92.
Tveita A, Fauskanger M, Bogen B, Haabeth OA. Tumor-specific CD4+ T cells eradicate myeloma cells genetically deficient in MHC class II display. Oncotarget. 2016;7:67175–82.
Savage ND, de Boer T, Walburg KV, Joosten SA, van Meijgaarden K, Geluk A, Ottenhoff TH. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol. 2008;181:2220–6.
Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2796–801.
Lanciotti M, Masieri L, Raspollini MR, Minervini A, Mari A, Comito G, et al. The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. Biomed Res Int. 2014. https://doi.org/10.1155/2014/486798.
Bostrom MM, Irjala H, Mirtti T, Taimen P, Kauko T, Algars A, et al. Tumor-associated macrophages provide significant prognostic information in urothelial bladder cancer. PLoS ONE. 2015;10:e0133552.
Dannenmann SR, Thielicke J, Stockli M, Matter C, von Boehmer L, Cecconi V, et al. Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology. 2013;2:e23562.
Lu J, Xu Y, Wu Y, Huang XY, Xie JW, Wang JB, et al. Tumor-infiltrating CD8+ T cells combined with tumor-associated CD68+ macrophages predict postoperative prognosis and adjuvant chemotherapy benefit in resected gastric cancer. BMC Cancer. 2019;19:920-z.
Salmi S, Siiskonen H, Sironen R, Tyynela-Korhonen K, Hirschovits-Gerz B, Valkonen M, et al. The number and localization of CD68+ and CD163+ macrophages in different stages of cutaneous melanoma. Melanoma Res. 2019;29:237–47.
Malesci A, Bianchi P, Celesti G, Basso G, Marchesi F, Grizzi F, et al. Tumor-associated macrophages and response to 5-fluorouracil adjuvant therapy in stage III colorectal cancer. Oncoimmunology. 2017;6:e1342918.
Acknowledgements
The authors thank Prof. A Irisawa, Prof. T Yazawa, and Prof. M Masuda for their advice. The authors also thank Mrs. C Matsuyama and Ms. A Shimizu for their technical assistance with IHC staining.
Author information
Authors and Affiliations
Contributions
HK and TJ contributed equally to this work; AA collected clinical information; HK and TJ reviewed the pathological diagnosis; HK and TJ analyzed the data and wrote the manuscript; RY, AK, OH, and AM made critical revisions to the manuscript; HK and TJ designed the study; HK gave the final approval of the manuscript for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential competing interests.
Ethical approval
The authors adhered to institutional ethical standards.
Informed consent
All patients signed an informed consent form for research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuroda, H., Jamiyan, T., Yamaguchi, R. et al. Tumor microenvironment in triple-negative breast cancer: the correlation of tumor-associated macrophages and tumor-infiltrating lymphocytes. Clin Transl Oncol 23, 2513–2525 (2021). https://doi.org/10.1007/s12094-021-02652-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-021-02652-3