Abstract
Purpose
To evaluate the value of multiparametric magnetic resonance imaging (MRI) in pretreatment prediction of breast cancers insensitive to neoadjuvant chemotherapy (NAC).
Methods
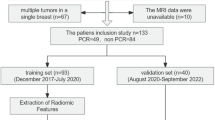
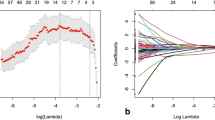
A total of 125 breast cancer patients (63 in the primary cohort and 62 in the validation cohort) who underwent MRI before receiving NAC were enrolled. All patients received surgical resection, and Miller–Payne grading system was applied to assess the response to NAC. Grade 1–2 cases were classified as insensitive to NAC. We extracted 1941 features in the primary cohort. After feature selection, the optimal feature set was used to construct a radiomic signature using machine learning. We built a combined prediction model incorporating the radiomic signature and independent clinical risk factors selected by multivariable logistic regression. The performance of the combined model was assessed with the results of independent validation.
Results
Four features were selected for the construction of the radiomic signature based on the primary cohort. Combining with independent clinical factors, the combined prediction model for identifying the Grade 1–2 group reached a better discrimination power than the radiomic signature, with an area under the receiver operating characteristic curve of 0.935 (95% confidence interval 0.848–1) in the validation cohort, and its clinical utility was confirmed by the decision curve analysis.
Conclusion
The combined model based on radiomics and clinical variables has potential in predicting drug-insensitive breast cancers.




Similar content being viewed by others
Abbreviations
- NAC:
-
Neoadjuvant chemotherapy
- MRI:
-
Magnetic resonance imaging
- HER2:
-
Human epidermal growth factor receptor-2
- DCE:
-
Dynamic contrast enhanced
- T2WI:
-
T2-weighted imaging
- DWI:
-
Diffusion-weighted imaging
- TR:
-
Repetition time
- TE:
-
Echo time
- FOV:
-
Field of view
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- ISH:
-
In situ hybridization
- LASSO:
-
Least absolute shrinkage and selection operator
- AUC:
-
Area under the receiver operating characteristic curve
- ROC:
-
Receiver operating characteristic
- GOF:
-
Goodness-of-fit
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
References
Kaufmann M, von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003;21(13):2600–8. https://doi.org/10.1200/jco.2003.01.136.
Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24(12):1940–9. https://doi.org/10.1200/jco.2005.02.6187.
Berruti A, Generali D, Kaufmann M, Puztai L, Curigliano G, Aglietta M, et al. International expert consensus on primary systemic therapy in the management of early breast cancer: highlights of the fourth symposium on primary systemic therapy in the management of operable breast cancer, Cremona, Italy (2010). J Natl Cancer Inst Monogr. 2011;2011(43):147–51. https://doi.org/10.1093/jncimonographs/lgr037.
Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18(12):1927–34. https://doi.org/10.1093/annonc/mdm201.
Mamounas EP. Neoadjuvant chemotherapy for operable breast cancer: is this the future? Clin Breast Cancer. 2003;4(Suppl 1):S10–9.
Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85. https://doi.org/10.1200/jco.2007.15.0235.
Iotti V, Ravaioli S, Vacondio R, Coriani C, Caffarri S, Sghedoni R, et al. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: a comparison with breast magnetic resonance imaging. Breast Cancer Res. 2017;19(1):106.
Le-Petross H, Lim B. Role of MR Imaging in Neoadjuvant Therapy Monitoring. Magn Reson Imaging Clin N Am. 2018;26(2):207–20.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16.
Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast (Edinb). 2003;12(5):320–7.
Untch M, Konecny GE, Paepke S, von Minckwitz G. Current and future role of neoadjuvant therapy for breast cancer. Breast (Edinb). 2014;23(5):526–37. https://doi.org/10.1016/j.breast.2014.06.004.
Babyshkina N, Malinovskaya E, Patalyak S, Bragina O, Tarabanovskaya N, Doroshenko A, et al. Neoadjuvant chemotherapy for different molecular breast cancer subtypes: a retrospective study in Russian population. Med Oncol. 2014;31(9):165. https://doi.org/10.1007/s12032-014-0165-7.
Shen C, Liu Z, Guan M, Song J, Lian Y, Wang S, et al. 2D and 3D CT radiomics features prognostic performance comparison in non-small cell lung cancer. Transl Oncol. 2017;10(6):886–94. https://doi.org/10.1016/j.tranon.2017.08.007.
Guo J, Liu Z, Shen C, Li Z, Yan F, Tian J, et al. MR-based radiomics signature in differentiating ocular adnexal lymphoma from idiopathic orbital inflammation. Eur Radiol. 2018;28(9):3872–3881. https://doi.org/10.1007/s00330-018-5381-7.
Liu Z, Wang Y, Liu X, Du Y, Tang Z, Wang K, et al. Radiomics analysis allows for precise prediction of epilepsy in patients with low-grade gliomas. NeuroImage Clin. 2018;19:271–8. https://doi.org/10.1016/j.nicl.2018.04.024.
Huang YQ, Liang CH, He L, Tian J, Liang CS, Chen X, et al. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J Clin Oncol. 2016;34(18):2157–64. https://doi.org/10.1200/jco.2015.65.9128.
Shen C, Liu Z, Wang Z, Guo J, Zhang H, Wang Y, et al. Building CT radiomics based nomogram for preoperative esophageal cancer patients lymph node metastasis prediction. Transl Oncol. 2018;11(3):815–24. https://doi.org/10.1016/j.tranon.2018.04.005.
Liu Z, Zhang XY, Shi YJ, Wang L, Zhu HT, Tang Z, et al. Radiomics Analysis for Evaluation of Pathological Complete Response to Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Clin Cancer Res. 2017;23(23):7253–62. https://doi.org/10.1158/1078-0432.ccr-17-1038.
Chamming’s F, Ueno Y, Ferre R, Kao E, Jannot AS, Chong J, et al. Features from computerized texture analysis of breast cancers at pretreatment mr imaging are associated with response to neoadjuvant chemotherapy. Radiology. 2018;286(2):412–20. https://doi.org/10.1148/radiol.2017170143.
Braman NM, Etesami M, Prasanna P, Dubchuk C, Gilmore H, Tiwari P, et al. Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res BCR. 2017;19(1):57. https://doi.org/10.1186/s13058-017-0846-1.
Verma V, Simone CB 2nd, Krishnan S, Lin SH, Yang J, Hahn SM. The rise of radiomics and implications for oncologic management. J Natl Cancer Inst. 2017. https://doi.org/10.1093/jnci/djx055.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–62. https://doi.org/10.1038/nrclinonc.2017.141.
Fujii T, Kogawa T, Dong W, Sahin AA, Moulder S, Litton JK, et al. Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer. Ann Oncol. 2017;28(10):2420–8. https://doi.org/10.1093/annonc/mdx397.
Omarini C, Guaitoli G, Noventa S, Andreotti A, Gambini A, Palma E, et al. Impact of time to surgery after neoadjuvant chemotherapy in operable breast cancer patients. Eur J Surg Oncol. 2017;43(4):613–8. https://doi.org/10.1016/j.ejso.2016.09.020.
Sauerbrei W, Royston P, Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26(30):5512–28.
Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. https://doi.org/10.1186/1472-6947-8-53.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Zhu Q, Tannenbaum S, Kurtzman SH, DeFusco P, Ricci A Jr, Vavadi H, et al. Identifying an early treatment window for predicting breast cancer response to neoadjuvant chemotherapy using immunohistopathology and hemoglobin parameters. Breast Cancer Res BCR. 2018;20(1):56. https://doi.org/10.1186/s13058-018-0975-1.
Liu YH, Ye JM, Xu L, Huang QY, Zhao JX, Duan XN, et al. Effectiveness of dynamic contrast-enhanced magnetic resonance imaging in evaluating clinical responses to neoadjuvant chemotherapy in breast cancer. Chin Med J. 2011;124(2):194–8.
Chen S, Liu Y, Ouyang QW, Huang L, Luo RC, Shao ZM. Clinical and pathological response to neoadjuvant chemotherapy based on primary tumor reduction is correlated to survival in hormone receptor-positive but not hormone receptor-negative locally advanced breast cancer. Ann Surg Oncol. 2015;22(1):32–9. https://doi.org/10.1245/s10434-014-3894-0.
von Minckwitz G, Untch M, Nüesch E, Loibl S, Kaufmann M, Kümmel S, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2011;125(1):145–56.
Lips EH, Mulder L, de Ronde JJ, Mandjes IA, Koolen BB, Wessels LF, et al. Breast cancer subtyping by immunohistochemistry and histological grade outperforms breast cancer intrinsic subtypes in predicting neoadjuvant chemotherapy response. Breast Cancer Res Treat. 2013;140(1):63–71. https://doi.org/10.1007/s10549-013-2620-0.
Zhu T, Liu CL, Zhang YF, Liu YH, Xu FP, Zu J, et al. A phase II trial of dose-dense (biweekly) paclitaxel plus carboplatin as neoadjuvant chemotherapy for operable breast cancer. Breast Cancer Res Treat. 2016;156(1):117–24. https://doi.org/10.1007/s10549-016-3735-x.
Acknowledgements
We thank all the patients for their participation and their physicians for their remarkable efforts. This study was funded by the National Key R&D Program of China [Grant No.: 2017YFC1309100]; Natural Science Foundation of Guangdong Province, China [grant numbers: 2017A030313882]; National Natural Science Foundation of China [Grant Nos.: 81871513, 81772012, 81501549]; the Beijing Natural Science Foundation [Grant No.: 7182109]; Science and Technology Planning Project of Guangdong Province [Grant No.: 2017B020227012]; and CSC0-constant Rui Tumor Research Fund, China [Grant No.: Y-HR2016-067].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that none of the authors have any conflict of interest.
Ethical approval
All procedures performed in this study, which involved human participants, were in accordance with the ethical standards of the institutional and/or nation research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xiong, Q., Zhou, X., Liu, Z. et al. Multiparametric MRI-based radiomics analysis for prediction of breast cancers insensitive to neoadjuvant chemotherapy. Clin Transl Oncol 22, 50–59 (2020). https://doi.org/10.1007/s12094-019-02109-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02109-8