Abstract
Purpose
6-phosphogluconate dehydrogenase (6PGD), a key enzyme of the oxidative pentose phosphate pathway, is involved in tumor growth and metabolism. Although high 6PGD activity has been shown to be associated with poor prognosis, its role and therapeutic value in breast cancer remain unknown.
Methods
The levels and roles of 6PGD were analyzed in breast cancer cells and their normal counterparts. The underlying mechanisms of 6PGD’s roles are also analyzed.
Results
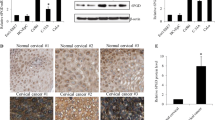
We found that 6PGD is aberrantly activated in breast cancer as shown by its increased transcriptional and translational levels as well as enzyme activity in breast cancer tissues and cell lines compared to normal counterparts. Although similar degree of enzyme activity inhibition was achieved in both breast cancer and normal breast cells, 6PGD inhibition by siRNA-mediated knockdown or pharmacological inhibitor physcion is more effective in inhibiting growth and survival in breast cancer than normal breast cells. Moreover, inhibiting 6PGD significantly sensitizes breast cancer response to chemotherapeutic agents in in vitro cell culture system and in vivo xenograft breast cancer model. We further show that 6PGD inhibition activates AMPK and its downstream substrate ACC1, leading to reduction of ACC1 activity and lipid biosynthesis. AMPK depletion significantly reverses the inhibitory effects of physcion in breast cancer cells, confirming that 6PGD inhibition targets breast cancer cell via AMPK activation.
Conclusions
Our work provides experimental evidence on the association of 6PGD with poor prognosis in breast cancer and suggests that 6PGD inhibition may represent a potential therapeutic strategy to augment chemotherapy efficacy in breast cancer.




Similar content being viewed by others
References
Dubey AK, Gupta U, Jain S. Breast cancer statistics and prediction methodology: a systematic review and analysis. Asian Pac J Cancer Prev. 2015;16(10):4237–45.
Gradishar WJ. Treatment challenges for community oncologists treating postmenopausal women with endocrine-resistant, hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. Cancer Manag Res. 2016;8:85–94. https://doi.org/10.2147/CMAR.S98249.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–74. https://doi.org/10.1073/pnas.191367098.
Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. https://doi.org/10.1038/nrc2981.
Bravard A, Luccioni C, Muleris M, Lefrancois D, Dutrillaux B. Relationships between UMPK and PGD activities and deletions of chromosome 1p in colorectal cancers. Cancer Genet Cytogenet. 1991;56(1):45–56.
Jonas SK, Benedetto C, Flatman A, Hammond RH, Micheletti L, Riley C, et al. Increased activity of 6-phosphogluconate dehydrogenase and glucose-6-phosphate dehydrogenase in purified cell suspensions and single cells from the uterine cervix in cervical intraepithelial neoplasia. Br J Cancer. 1992;66(1):185–91.
Giusti L, Iacconi P, Ciregia F, Giannaccini G, Donatini GL, Basolo F, et al. Fine-needle aspiration of thyroid nodules: proteomic analysis to identify cancer biomarkers. J Proteome Res. 2008;7(9):4079–88. https://doi.org/10.1021/pr8000404.
Lin R, Elf S, Shan C, Kang HB, Ji Q, Zhou L, et al. 6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling. Nat Cell Biol. 2015;17(11):1484–96. https://doi.org/10.1038/ncb3255.
Shan C, Elf S, Ji Q, Kang HB, Zhou L, Hitosugi T, et al. Lysine acetylation activates 6-phosphogluconate dehydrogenase to promote tumor growth. Mol Cell. 2014;55(4):552–65. https://doi.org/10.1016/j.molcel.2014.06.020.
Chan B, VanderLaan PA, Sukhatme VP. 6-Phosphogluconate dehydrogenase regulates tumor cell migration in vitro by regulating receptor tyrosine kinase c-Met. Biochem Biophys Res Commun. 2013;439(2):247–51. https://doi.org/10.1016/j.bbrc.2013.08.048.
Zheng W, Feng Q, Liu J, Guo Y, Gao L, Li R, et al. Inhibition of 6-phosphogluconate dehydrogenase reverses cisplatin resistance in ovarian and lung cancer. Front Pharmacol. 2017;8:421. https://doi.org/10.3389/fphar.2017.00421.
Elf S, Lin R, Xia S, Pan Y, Shan C, Wu S, et al. Targeting 6-phosphogluconate dehydrogenase in the oxidative PPP sensitizes leukemia cells to antimalarial agent dihydroartemisinin. Oncogene. 2017;36(2):254–62. https://doi.org/10.1038/onc.2016.196.
Brocklehurst D, Champion AE, Cheek TR, Dewhurst DG. The value of 6-phosphogluconate dehydrogenase (6-PGDH) activity as a marker of tumour cellularity and prognostic indicator in primary breast cancer. Tumour Biol. 1986;7(2–3):99–104.
Baillet A, Xu R, Grichine A, Berthier S, Morel F, Paclet MH. Coupling of 6-phosphogluconate dehydrogenase with NADPH oxidase in neutrophils: Nox2 activity regulation by NADPH availability. FASEB J. 2011;25(7):2333–43. https://doi.org/10.1096/fj.10-173807.
Shirra MK, Patton-Vogt J, Ulrich A, Liuta-Tehlivets O, Kohlwein SD, Henry SA, et al. Inhibition of acetyl coenzyme A carboxylase activity restores expression of the INO1 gene in a snf1 mutant strain of Saccharomyces cerevisiae. Mol Cell Biol. 2001;21(17):5710–22.
Soule HD, Maloney TM, Wolman SR, Peterson WD Jr, Brenz R, McGrath CM, et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50(18):6075–86.
Briand P, Petersen OW, Van Deurs B. A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. Vitro Cell Dev Biol. 1987;23(3):181–8.
Fogarty S, Hardie DG. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta. 2010;1804(3):581–91. https://doi.org/10.1016/j.bbapap.2009.09.012.
Ha J, Daniel S, Broyles SS, Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem. 1994;269(35):22162–8.
Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7(10):791–9. https://doi.org/10.1038/nrc2212.
Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292(5516):504–7. https://doi.org/10.1126/science.1058079.
Sukhatme VP, Chan B. Glycolytic cancer cells lacking 6-phosphogluconate dehydrogenase metabolize glucose to induce senescence. FEBS Lett. 2012;586(16):2389–95. https://doi.org/10.1016/j.febslet.2012.05.052.
Acknowledgements
This work was supported by a research grant provided by Hubei Provincial Health and Family Planning Commission (Grant No. 2016-2017 15).
Author information
Authors and Affiliations
Contributions
XYY and JRH designed the experiments, interpreted the data and wrote the manuscript. XYY, XCP and JRH performed the experiments. All authors approved the final manuscripts.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interests.
Informed consent
Breast cancer samples were obtained from patients seen at the Xiangyang No.1 People’s Hospital. Written informed consent was obtained from all patients under institutional review board-approved protocols.
Research involving human participants and/or animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, X., Peng, X. & Huang, J. Inhibiting 6-phosphogluconate dehydrogenase selectively targets breast cancer through AMPK activation. Clin Transl Oncol 20, 1145–1152 (2018). https://doi.org/10.1007/s12094-018-1833-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1833-4