Abstract
Localized rectal adenocarcinoma is a heterogeneous disease and current treatment recommendations are based on a preoperative multidisciplinary evaluation. High-resolution magnetic resonance imaging and endoscopic ultrasound are complementary to do a locoregional accurate staging. Surgery remains the mainstay of treatment and preoperative therapies with chemoradiation (CRT) or short-course radiation (SCRT) must be considered in more locally advanced cases. Novel strategies with induction chemotherapy alone or preceding or after CRT (SCRT) and surgery are in development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer represents a major health problem in developed countries. In Spain, 32,240 new cases were diagnosed (15% of global cancer incidence) and 14,700 patients died (14% of total cancer mortality) in 2012. Approximately 35% of these tumors arise in the rectum. The incidence increases with age. Median age at diagnosis is about 70 years. Other risk factors include diet (red or processed meat excess), tobacco and alcohol. Type II diabetes, ulcerative colitis and Crohn’s disease also increase the risk. The hereditary component is less pronounced for rectal cancer than for colon cancer. The Spanish Society of Medical Oncology (SEOM) invited ten experts based on major scientific contribution in the field. The purpose of this paper was to define current “state of the art” using the methodology of evidence-based medicine. The available medical literature was reviewed according to main topics of disease management, and classified by scientific levels of evidence and grades of clinical recommendation (Table 1) [1]. The resulting text was reviewed, discussed, and approved by all authors.
Diagnosis
Although clear definition of rectal cancer is lacking, it is usually defined as a tumor with its lower edge within 12–15 cm from the anal verge measured by rigid sigmoidoscopy or MRI.
Adenocarcinoma is the most common histology (95–98%), other unusual tumors as melanomas, gastrointestinal stromal tumors or neuroendocrine tumors are not included in this guideline.
Staging
Clinical stage is critical to stablish locoregional extension of the disease and risk of recurrence to individualize neoadjuvant treatment strategies in a multidisciplinary team. It requires a physical examination, a complete blood count, liver and renal function test, and carcinoembryonic antigen. A total colonoscopy to evaluate for synchronous lesions and a rigid sigmoidoscopy to determinate the location of the tumor must be performed. If the tumor is obstructive, virtual colonoscopy is recommended (but complete colonoscopy should be performed after surgery).
Both endorectal ultrasound (ERUS) and rectal magnetic resonance imaging (MRI) are recommended for early rectal cancer staging (cT1–T2), and high-resolution MRI has become the standard method for evaluating locally advanced disease particularly those patients with potential CRM involvement [2, 3]. MRI-based pretherapeutic definition of an involved CRM is an independent prognostic factor for 5-year overall survival, disease-free survival and for local recurrence [4]. MRI-defined extramural venous invasion (EMVI) is an additional independent poor prognostic factor for both local recurrence and for DFS in stage II/III rectal cancer [5]. A reliable assessment of the pretherapeutic mesorectal regional lymph node status is not possible by the present imaging modalities (Table 2 shows comparison between imaging methods.) Although surgery is the standard of care after neoadjuvant treatment, identification of complete response is interesting to detect patients who could potentially undergo organ-preserving treatment. Post neoadjuvant treatment MRI is the most studied method. The MERCURY study validated the use of high-resolution MRI for posttreatment staging and its correlation with survival outcomes (modified Mandard grading system) [6]. Some groups suggest using diffusion-weighted (DW) MRI to increase accuracy of tumor response assessment [7].
Contrast-enhanced computed tomography (CT) scan of the chest, abdomen and pelvic should be carried out to estimate distant spread of the disease. Chest X-ray and abdominal MRI should be considered if CT is contraindicated.
The 7th edition of the TNM staging system should be used for clinical and histopathological staging. T1 tumors could also be classified according to Haggitt and Kikuchi stages depending on polyp morphology.
Pathological staging should include the quality of mesorectum, proximal, distal and circumferential margins (a positive CMR is defined as tumor within 1 mm from the margin), lymphovascular and nerve invasion, number of regional lymph nodes and extranodal tumor deposits and effect of neoadjuvant treatment.
Management of local disease
Early rectal cancer: cT1/cT2 and cN0
Oncologic outcome after rectal resection for early rectal cancer (T1–2, N0) has been proved good. Nevertheless, radical rectal surgery (RRS) implies a temporary or definitive stoma, low anterior resection syndrome and sexual and urinary dysfunction. That is why some new approaches have been proposed.
Transanal endoscopic microsurgery (TEM) has shown its superiority over local resection [8]. Two meta-analyses have compared the efficacy of TEM and total mesorectal excision for the treatment of T1 rectal cancer. Although there were not differences in distant metastases rates and overall survival, a higher risk of local recurrence was evidenced [9, 10]. Similarly, data from Norwegian Colorectal Cancer Registry show the absence of differences in 5-year relative survival rate but a significant lower 5-year local recurrence rate after total mesorectal excision [11].
Due to high local recurrence rates, TEM is not a proper technique for all patients with T1 rectal cancer but is useful for patients with low-risk T1 tumors (low tumor grade, absence of lymphovascular invasion and clear margin of resection) [12].
For T2N0 rectal cancer, total mesorectal excision is the standard of care. TEM exclusive treatment leads to inacceptable local recurrence rates. However, some alternative has been proposed in two recent trials. In phase II trial ACOSOG Z6041, selected T2N0 rectal tumors received radiotherapy combined with capecitabine plus oxaliplatin followed by local excision. After a mean follow-up of 56 months, local recurrence rate was 4%. 3- and 5-year disease-free survival for the per-protocol group was 86.9 and 80.3% [13]. These findings suggest that neoadjuvant chemoradiotherapy plus local TEM might be an alternative to radical surgery in selected patients with distal rectal cancer seeking organ-preserving surgery to avoid a permanent stoma.
Resectable locally advanced rectal cancer: cT3–T4 any cN+
Surgery: open and laparoscopic
Laparoscopic surgery was first considered in 1990 for patients undergoing colectomy for cancer. Based on reports of tumor recurrences within surgical wounds, there was a concern that this approach would compromise oncologic outcome by failing to achieve a proper oncologic resection or by altering patterns of recurrence. Since then, some trials have been carried out to clear up this issue.
In rectal cancer, results of randomized trial COLOR II showed a 3-year locoregional recurrence rate of 5.0% in the two, a disease-free survival rates of 74.8% in the laparoscopic-surgery group and 70.8% in the open-surgery group, as well as absence of differences in overall survival rates (86.7 versus 83.6%) [14]. Nowadays, there was no doubt regarding the benefit in postoperative recovery of the laparoscopic approach for colorectal cancer and its equivalence in oncologic outcome.
Preoperative chemoradiation (CRT) or short-course radiotherapy (SCRT)
Two different preoperative approaches have been established for cStage II and III patients.
One is preoperative chemoradiation (CRT) because the german study demonstrated less toxicity and better locoregional outcomes with preoperative versus postoperative chemoradiotherapy [15]. Two studies adding concomitant 5FU or capecitabine to preoperative radiation therapy (45–50.4 Gy in 25–28 fractions) have demonstrated better local control with increased pathological responses and lower rates of local recurrences with respect to radiation therapy alone. No significant differences were observed in overall survival in these studies [16, 17]. Concomitant capecitabine is not inferior to 5FU and is standard nowadays [18, 19]. Adding oxaliplatin or targeted drugs to fluoropyrimidines do not improve outcomes and are not recommended although in some studies higher rates of pathological responses are achieved [20, 21].
The second standard approach, preoperative short-course radiotherapy (SCRT), 25 Gy over 5 days without concomitant chemotherapy, has demonstrated decreased rate of local recurrences compared to surgery alone [22].
Two randomized studies comparing both strategies (CRT versus SCRT) do not show differences in rate of local recurrence and survival. Differences in tumor downstaging were observed in favor of CRT [23, 24], so more locally advanced rectal cancers (T4/mesorectal fascia affected or close) should be treated with preoperative chemoradiation.
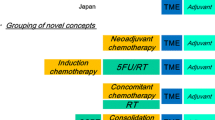
Total neoadjuvant treatment (Induction chemotherapy followed by CRT)
Induction CT may be associated with better treatment compliance and may allow full systemic doses of chemotherapy to be delivered. The timeline in the current standard treatment sequence means that full systemic chemotherapy is not delivered until about 4 months after neoadjuvant CRT is initiated.
The PAN-EX analysis showed that intensification of systemic therapy with neoadjuvant combination CT before standard treatment was feasible in poor-risk potentially operable rectal cancer, with acceptable safety and promising outcomes [25].
The Spanish GCR-3 phase II randomized trial compared preoperative CRT, followed by surgery and adjuvant CT with induction CT followed by CRT and surgery. There were no significant differences between the two arms in pCR while at the same time achieving more favorable compliance and toxicity profiles with induction versus adjuvant chemotherapy. At 5 years, there were no differences in cumulative incidence of local relapse, incidence of distant metastases DFS or OS [26].
Maréchal et al. [27] also compared standard therapy with induction FOLFOX, followed by CRT and then surgery. No differences in pCR were noted. The other four studies were single-arm trials, and the pCR rates ranged from 20% [28, 29] to 33% (Table 3).
Other potential advantages of the total neoadjuvant approach are: rapid relief of symptom such as rectal pain or bleeding and substantially shorter time to temporary ostomy reversal. A limitation of the induction strategy is overtreatment. So preoperative high-resolution MRI is mandatory in the selection of patients. In summary, the use of induction chemotherapy prior to neoadjuvant CRT in patients with rectal cancer can be proposed as an optional alternative in high-risk patients, but more data are needed on the use of induction strategies to know the impact on survival outcomes.
Adjuvant therapy
The administration of systemic adjuvant chemotherapy after preoperative chemoradiation and total mesorectal excision is controversial. Four randomized trials specifically addressing its benefit have faced with considerable challenges (old 5-FU-based schemes, poor patient accrual and compliance) and have not demonstrated its superiority over observation [30–33]. Notwithstanding this paucity of evidence, two randomized studies have compared oxaliplatin with nonoxaliplatin-containing regimens for adjuvant therapy after chemoradiation and surgery of rectal cancer [34, 21]. These two trials found moderate (4.7% and 8.7%) increase in 3-year DFS. In practice, adjuvant chemotherapy has become a widespread practice based mainly on extrapolation from results in colon cancer.
Are there any subgroups who may benefit from adjuvant chemotherapy?
Several studies have suggested that not all patients with rectal cancer benefit from adjuvant chemotherapy. The degree of bowel wall penetration and nodal involvement at pathology examination has been shown to be the most important predictors of long-term outcome. There is compelling evidence that patients downstaged to ypT0–2N0 after chemoradiation have very favorable prognoses, particularly in the setting of high-quality preoperative imaging and surgical technique, even in the absence of systemic treatment [35]. Even though patients with pCR seem likely to have a better outcome than do those without pCR, whether improvement in pCR in clinical trials is a useful means to select treatments that are likely to result in better survivals is unclear [36]. In contrast, node positive patients carry on significantly worse prognoses as in colon cancer.
Recommendation For patients treated with preoperative chemoradiotherapy, decisions about postoperative systemic treatment could be tailored to the risk of metastatic disease (based on pathology), taking also into account patient’s comorbidities, life expectancy, and preferences. They should be informed about the uncertain benefit of adjuvant chemotherapy (II, B):
-
(A)
ypT0N0 patients must be informed about their good intrinsic prognosis and the possibility to be followed up without any more treatment.
-
(B)
ypT1–2N0 patients can be treated with infusional 5-FU, capecitabine monotherapy or standard oxaliplatin-based chemotherapy (to complete 6 months of treatment).
-
(C)
Most patients with ypT3-4 or N + should be offered oxaliplatin-based chemotherapy (FOLFOX × 8 or XELOX × 4–5 courses).
-
(D)
for patients who are frail, with significant comorbidities, or with life expectancy of less than 5 years, chemotherapy should be omitted.
Adjuvant treatment after immediate radical standard surgery (TME)
Postoperative chemoradiotherapy with concomitant fluoropyrimidine-based chemotherapy is no longer recommended. However, it could be used in patients with positive CRM, perforation in the tumor area, defects in the mesorectum, or in other cases with high risk of local recurrence. As in stage III and high-risk colon cancer, adjuvant chemotherapy can be given, even though the level of evidence for benefit is lower in rectal cancer.
Unresectable disease: cT4
Unresectable rectal cancer constitutes 10–15% of primary rectal cancers, due to high risk of non-radical surgery or local failure. Without an uniform definition of unresectable rectal cancer, one of these would be a tumor palpably fixed to adjacent non-resectable structures such as the proximal sacrum, pelvic sidewall, pelvic floor, prostate, or base of the urinary bladder. But the use of radiological MRI-based criteria is recommended.
In the non-resectable cases, preoperative ChemoRT was used early to sterilize peripherally located disease and induce tumor shrinkage to allow subsequent radical surgery. Fluoropyrimidines as a chemotherapy and total doses of 50.4 Gy are the standard. Some studies with 66 in 2 Gy fractions or more without compromising organs at risk have been published [37].
At the moment, there is one randomized trial comparing RT versus ChemoRT (fluoropyrimidines), and the results are significative in favor ChemoRT [38]. The results adding oxaliplatin at the treatment schedule were disappointing, and oxaliplatin is used only in some cases in the induction setting [39].
Recommendations Chemo/RT (Capecitabine/long-course RT or infusional 5-FU/long-course RT) or induction chemotherapy (FOLFOX or CapeOx or 5-FU/leucovorin or capecitabine) followed by CRT.
When surgery is performed without an effective result, active chemotherapy regimen for advanced disease is mandatory.
Follow-up
Despite potentially curative surgery including total mesorectal excision and the use of modern neoadjuvant chemoradiotherapy and adjuvant chemotherapy and/or radiation therapy (RT), about 30% of patients who present stage II or III disease will have a disease recurrence following primary therapy.
Intensive follow-up strategies are associated with a shorter time in detecting recurrences, increase the detection of asymptomatic recurrences and the proportion of patients who are potentially eligible for curative therapy. A survival benefit from such an approach has in fact been shown in several meta-analyses. According to those meta-analyses, prospective randomized clinical studies and most international groups, we recommend intensive postoperative surveillance for stage II–III patients who have undergone a curative resection of rectal cancer with or without neoadjuvant chemoradiation therapy [40, 41].
Recommendation (Table 4)
-
A clinical encounter with a physician every 3 to 6 months for the first 3 years, and every 6 months during years 4 and 5.
-
Serum carcinoembryonic antigen (CEA) level at each follow-up visit for at least the first 3 years.
-
Annual computed tomography of the chest, abdomen, and pelvis for 5 years.
-
Full colonoscopy one year after surgery to exclude new lesions, or 3–6 months after surgery if colon not preoperatively complete studied. Further colonoscopy frequency depends on the results of the 1-year colonoscopy, with repeat examination in 3 years for patients without adenomas and 1 year for patients with adenomas. Patients at higher risk for local recurrence may be considered for proctosigmoidoscopy every six months for three to five years. Higher-risk patients may include those who have undergone low anterior resection and not received pelvic radiation therapy, those with poorer-risk tumors (T2 or poor differentiation) who underwent local excision, those with positive margins (≤1 mm), and those with T4 or N2 rectal cancers.
Management of local recurrence
Despite improvements in preoperative selection better treatment strategies and optimal surgery, about 10% of patients with rectal cancer will develop locoregional relapse. The management of these patients is particularly challenging.
Nearly half of locally recurrent rectal cancers (LRRC) are located in the pelvis without distant metastasis. The best treatment for LRRC in this setting is a complete resection of the recurrent tumor [42].
However, the majority of patients develop recurrence involving the pelvic wall. Extended surgery such as abdomino-sacral resection has not been popular because 5-year survival rates are low and significant postoperative morbidity. In these patients, multimodality therapy including radical surgery and chemotherapy, radiotherapy or intraoperative radiotherapy (IORT) have been reported with 5-year survival of up to 31% and local control rates of 50–71% [43, 44].
For patients with non-fixed recurrent tumors who had not received previously radiation therapy (RT), appropriate treatment would a preoperative CRT approach followed by surgical intervention. If the tumor is fixed to adjacent structures, then preoperative treatment including induction chemotherapy, chemoradiation followed by re-evaluation for surgery is the most appropriate treatment option [45, 46].
For patients with locally recurrent rectal cancer following high-dose pelvic radiation, management decisions have generally been directed toward palliative care or chemotherapy alone. Although historically considered unsafe, reirradiation in the pelvis has been investigated, and today in patients with recurrent rectal cancer who have received prior RT, the treatment with reduced-dose RT, either given daily or hyperfractionated, combined or not with chemotherapy and re-evaluation for resection could be an option. Many studies have reported until 40% 5-year overall survival rate, 60% overall response rate and 93% clinical response with a good or complete palliative effect on intractable pain and/or bleeding. The incidence of RTOG grade 3–4 toxicity was less than 10–23% [47].
Finally, intraoperative radiotherapy (IORT), either with electron beam or high-dose-rate brachytherapy, or stereotactic body radiotherapy (SBRT), could be used in selected centers with the appropriate experience with promising results.
Special situations
Rectal cancer and synchronous metastatic disease
Treatment strategy for synchronous oligometastatic rectal cancer should be based on the possibility of achieving R0 resection, either initially or after induction treatment for systemic disease and primary tumor.
Resectable
In locally advanced primary tumors (≥T3 or N+): local radiotherapy (short course) before of after preoperative oxaliplatin-based chemotherapy in combination with fluoropyrimidine (FOLFOX/CAPOX) for 3 months followed by resection of the primary (staged or synchronous) followed by postoperative FOLFOX/CAPOX for 3 months should be applied (7) [V, B].
In early primary tumors (<T3 N0): resection of primary and metastases followed by postoperative treatment with FOLFOX/CAPOX for a total of 6 months could be considered, and if necessary (e.g., CRM+, etc.) postoperative local treatment according to stage [V, B].
Unresectable
For initial unresectable metastatic disease, most active available induction systemic treatment should be chosen [IV, A]. If metastases become resectable, local treatment according to stage for primary followed by resection of primary and metastases should be performed, followed by postoperative continuation of the same regimen for a total of 6 months (including preoperative) [IV, A]. If metastases remain unresectable, treatment should be continued or switched, depending on the quality of response [V, B].
Never resectable metastatic disease: Treatment aim is palliation and chemotherapy should be chosen. Radical and mutilating surgery of the primary should be avoided, unless necessitated by an emergency situation [V, B].
In case of symptomatic primary of the rectum: local measures (e.g., insertion of a stent or stoma, radiotherapy) should be performed initially, and palliative surgical resection only in specific circumstances [V, B].
Future trends: preoperative chemotherapy only/consolidative CT after CRT or SCRT
Preoperative CT without RT is attractive because pre-operative pelvic radiation is associated with an increased incidence of serious late side effects, and data from retrospective analyses suggest that radiation may not be needed in some patients with T3 tumors. Two phase II trials in T3, middle third tumors have reported encouraging results (20–27% pCR; 100% R0 resections) with an induction combination of fluoropyrimidine/oxaliplatin and bevacizumab [48, 49]. In a similar population the phase II/III intergroup Prospect trial (NCT01515787) is now open and compares pre-operative chemotherapy with selective chemoradiation versus standard pre-operative CRT in a similar population.
Tumor response to RT is time-dependent, and longer intervals from RT to surgery and delivering systemic chemotherapy after chemoradiation rather than before might have a greater effect on tumor response [50]. In a non-randomized phase 2 trial with four sequential groups of patients with rectal cancer, adding up to six cycles of modified FOLFOX6 (mFOLFOX6) CT between preoperative CRT and surgery, increased the proportion of patients who achieved a pCR [51].
This strategy is being compared with induction CT followed by CRT and surgery in a randomized multi-institutional American trial. NCT02008656 [52].
A randomized phase 3 trial comparing SCRT followed by consolidation CT with FOLFOX4 versus long-course CRT, showed an improvement in 3-year overall survival with the first approach, without differences in pCR rate, local control or disease-free survival [53]. The acute toxicity was significantly lower in the SCRT arm. Another trial (RAPIDO) compares short-course RT followed by prolonged preoperative CT and surgery with standard CRT and surgery and optional adjuvant CT in high-risk rectal cancer [54].
References
Dykewicz CA. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001;33:139–44.
Balyasnikova S, Browm G. Optimal imaging strategies for rectal cancer staging and ongoing management. Curr Treat Options Oncol. 2016;17:32.
Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging meta-analysis. Radiology. 2004;232(3):773e83.
Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32(1):34–43.
Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95(2):229–36.
Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29(28):3753–60.
Maas M, Lambregts DM, Nelemans PJ, Heijnen LA, Martens MH, Leijtens JW, et al. Assessment of clinical complete response after chemoradiation for rectal cancer with digital rectal examination, endoscopy, and MRI: selection for organ-saving treatment. Ann Surg Oncol. 2015;22:3873–80.
Clancy C, Burke JP, Albert MR, O’Connell PR, Winter DC. Transanal endoscopic microsurgery versus standard transanal excision for the removal of rectal neoplasms: a systematic review and meta-analysis. Dis Colon Rectum. 2015;58(2):254–61. doi:10.1097/DCR.0000000000000309.
Lu JY, Lin GL, Qiu HZ, Xiao Y, Wu B, Zhou JL. Comparison of transanal endoscopic microsurgery and total mesorectal excision in the treatment of T1 rectal cancer: a meta-analysis. PLoS One. 2015;10(10):e0141427. doi:10.1371/journal.pone.0141427.
Sajid MS, Farag S, Leung P, Sains P, Miles WF, Baig MK. Systematic review and meta-analysis of published trials comparing the effectiveness of transanal endoscopic microsurgery and radical resection in the management of early rectal cancer. Colorectal Dis. 2014;16(1):2–14. doi:10.1111/codi.12474.
Stornes T, Wibe A, Nesbakken A, Myklebust TÅ, Endreseth BH. National early rectal cancer treatment revisited. Dis Colon Rectum. 2016;59(7):623–9. doi:10.1097/DCR.0000000000000591.
Junginger T, Goenner U, Hitzler M, Trinh TT, Heintz A, Wollschlaeger D, et al. Long-term oncologic outcome after transanal endoscopic microsurgery for rectal carcinoma. Dis Colon Rectum. 2016;59(1):8–15 PMID: 26651106.
Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015;16(15):1537–46. doi:10.1016/S1470-2045(15)00215-6.
Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange-de Klerk ES, COLOR II Study Group, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372(14):1324–32. doi:10.1056/NEJMoa1414882 PMID: 25830422.
Sauer R, Becker H, Hohengerger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Eng J Med. 2004;351:1731–40.
Gerard JO, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluoruracil and leucovorin in T3-4 rectal cancers: results of FFOD9203. J Clin Oncol. 2006;24:4620–5.
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23.
Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus fluoruracil for locally advanced rectal cancer: a randomized, multicentre, non-inferiority, phase 2 trial. Lancet Oncol. 2012;13:579–88.
O`Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, et al. Capecitabine and oxaliplatin in the preoperative multimodality tratment of rectal cancer:surgical end points from the National Surgical Adjuvant Breast and Bowel Proyect trial R-04. J Clin Oncol. 2014;32:1927–34.
Aschele C, Cioni L, Lonardi S, Pinto C, Cordio S, Rosati G, et al. Primary tumor response to preoperatively chemoradiation with or without oxaliplatin in locally advanced rectal cáncer: pathological results of the STAR01 randomized phase III trail. J CIin Oncol. 2011;29:2773–80.
Rodel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. Oxaliplatin added to fluoruracil-based preoperatively chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO 04-study): final results of the multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2015;16:979–89.
Van Gijn W, Marignen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorrectal excision for resectable rectal cáncer: 12 years follow-up of the multicentre, randomized controlled TME trial. Lancet Oncol. 2011;12:575–82.
Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–23.
Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, et al. Randomized trial of short-course radiotherapy versus long course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer. J Clin Oncol. 2012;30:3827–33.
Sclafani F, Brown G, Cunningham D, Wotherspoon A, Tait D, Peckitt C, et al. PAN-EX: a pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer. Ann Oncol. 2016;27(8):1557–65.
Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26:1722–8.
Maréchal R, Vos B, Polus M, Delaunoit T, Peeters M, Demetter P, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23:1525–30.
Perez K, Safran H, Sikov W, Vrees M, Klipfel A, Shah N, et al. Complete neoadjuvant treatment for rectal cancer: the Brown University Oncology Group CONTRE Study. Am J Clin Oncol. 2014. [Epub ahead of print].
Schou JV, Larsen FO, Rasch L, Linnemann D, Langhoff J, Høgdall E, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23:2627–33.
Bosset JF, CalaisG Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: ong-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–90.
Sainato A, CernuscoLunaNunzia V, Valentini V, De Paoli A, Maurizi ER, Lupattelli M, et al. No benefit of adjuvant Fluorouracil Leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): Long term results of a randomized trial (I-CNR-RT). Radiother Oncol. 2014;113(2):223–9.
Breugom AJ, van Gijn W, Muller EW, Berglund Å, van den Broek CB, Fokstuen T, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (Chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26:696–701.
Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, et al. Chronicle: results of a randomized phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomizing postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25:1356–62.
Hong YS, Nam BH, Kim KP, Kim JE, Park SJ, Park YS, et al. Oxaliplatin, fluorouracil, and leucovorin versus fluorouracil and leucovorin as adjuvant chemotherapy for locally advanced rectal cancer after preoperative chemoradiotherapy (ADORE): an open-label, multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2014;15:1245–53.
Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–44.
Maas M, Nelemans PJ, Valentini V, Crane CH, Capirci C, Rödel C, et al. Adjuvant chemotherapy in rectal cancer: defining subgroups who may benefit after neoadjuvant chemoradiation and resection: a pooled analysis of 3313 patients. Int J Cancer. 2015;137:212–20.
Caravatta L, Picardi V, Tambaro R, Padula GD, Macchia G, Deodato F, et al. Neoadjuvant accelerated concomitant boost radiotherapy and multidrug chemotherapy in locally advanced rectal cancer: a dose-escalation study. Am J Clin Oncol. 2012;35(5):424–31.
Braendengen M, Tveit KM, Berglund A, Birkemeyer E, Frykholm G, Pahlman L, et al. Randomized phase III study comparing preoperative radiotherapy with chemora- diotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26:3687–94.
Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Laffay I, Hennequin C, Etienne PL, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28(10):1638–44.
Steele SR, Chang GJ, Hendren S, Weiser M, Irani J, Buie WD, et al. Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. Practice guideline for the surveillance of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2015;58:713–25.
Pita-Fernández S, Alhayek-Aí M, González-Martín C, López-Calviño B, Seoane-Pillado T, Pértega-Díaz S. Intensive follow-up strategies improve outcomes in non metastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26:644–62.
Young PE, Womeldorph CM, Johnson EK, Maykel JA, Brucher B, Stojadinovic A, et al. Early detection of colorectal cancer recurrence in patients undergoing surgery with curative intent: current status and challenges. J Cancer. 2014;5:262–71.
Enríquez-Navascués JM, Aintzane Lizerazu NB, Placer C, Elosegui JL, Ciria JP, Lacasta A, et al. Patterns of local recurrence in rectal cancer after a multidisciplinary approach. World J Gastroenterol. 2011;17:1674–84.
Tanis PJ, Doeksen A, van Lanschot JJ. Intentionally curative treatment of locally recurrent rectal cancer: a systematic review. Can J Surg. 2013;56:135–44.
Lee JH, Kim DY, Kim SY, Park JW, Choi HS, Oh JH, et al. Clinical outcomes of chemoradiotherapy for locally recurrent rectal cancer. Radiat Oncol. 2011;6:51.
You YT, Chen JS, Wang JY, Tang R, Changchien CR, Chiang JM, et al. Concurrent chemoradiotherapy in the treatment of locally recurrent rectal cancer. Hepatogastroenterology. 2013;60:94–8.
Valentini V, Morganti AG, Gambacorta MA, Mohiuddin M, Doglietto GB, Coco C, et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: a multicentric phase II study. Int J Radiat Oncol Biol Phys. 2006;64:1129–39.
Fernandez-Martos C, Brown G, Estevan R, Salud A, Montagut C, Maurel J, et al. Preoperative chemotherapy in patients with intermediate-risk rectal adenocarcinoma selected by high- resolution magnetic resonance imaging: the GEMCAD 0801 Phase II Multicenter Trial. Oncologist. 2014;19:1042–3.
Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32(6):513–8.
Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a meta-analysis of published studies. Ann Surg. 2016;263:458–64.
Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957–66.
Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, et al. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;23(15):767.
Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Kryński J, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27:834–42.
Nilsson PJ, van Etten B, Hospers GA, Påhlman L, van de Velde CJ, Beets-Tan RG, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer—the RAPIDO trial. BMC Cancer. 2013;13:279. doi:10.1186/1471-2407-13-279.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that they have no potential conflicts of interest related to the publication of this manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
González-Flores, E., Losa, F., Pericay, C. et al. SEOM Clinical Guideline of localized rectal cancer (2016). Clin Transl Oncol 18, 1163–1171 (2016). https://doi.org/10.1007/s12094-016-1591-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-016-1591-0