Abstract
Purpose
To evaluate the efficacy and safety profile of the combination of panitumumab and irinotecan every 3 weeks in a phase II trial as second-line treatment in patients with advanced wild-type (WT) K-RAS colorectal cancer (CRC).
Methods
Fifty-three patients received 9 mg/kg of panitumumab followed by 350 mg/m2 of irinotecan every 21 days until disease progression, unacceptable toxicity or consent withdrawal.
Results
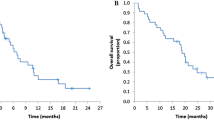
Median age of patients included was 67 years. All patients had previously received 5-fluorouracil, 84 % oxaliplatin and 8 % irinotecan as first-line treatment. Patients received a median of five infusions of panitumumab and irinotecan. On an intention-to-treat analysis, 12 patients (23 %) achieved partial responses and 22 patients (41 %) achieved disease stabilization. Median progression-free survival and overall survival were 4.5 and 15.1 months, respectively. The most frequent treatment-related severe toxicities per patient were diarrhoea (35.8 %), followed by skin rash (32.1 %), asthenia (18.9 %) and neutropenia (13.2 %). A significant association between clinical response and incidence and grade of skin toxicity was observed (p = 0.0032).
Conclusion
This study shows that the administration of panitumumab plus irinotecan every 3 weeks is safe, active and feasible as second-line treatment in patients with advanced WT K-RAS CRC.

Similar content being viewed by others
References
Folprecht G, Seymour MT, Saltz L et al (2008) Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol 26:1443–1451
Jonker DJ, O’Callaghan CJ, Karapetis CS et al (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357:2040–2048
Van Cutsem E, Peeters M, Siena S et al (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25:1658–1664
Karapetis CS, Khambata-Ford S, Jonker DJ et al (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765
Douillard JY, Siena S, Cassidy J et al (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28:4697–4705
Peeters M, Price TJ, Cervantes A et al (2010) Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28:4706–4713
Tabernero J, Ciardiello F, Rivera F et al (2010) Cetuximab administered once every second week to patients with metastatic colorectal cancer: a two-part pharmacokinetic/pharmacodynamic phase I dose-escalation study. Ann Oncol 21:1537–1545
Weiner LM, Belldegrun AS, Crawford J et al (2008) Dose and schedule study of panitumumab monotherapy in patients with advanced solid malignancies. Clin Cancer Res 14:502–508
Amado RG, Wolf M, Peeters M et al (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634
Cunningham D, Humblet Y, Siena S et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Fuchs CS, Moore MR, Harker G et al (2003) Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol 21:807–814
Haller DG, Rothenberg ML, Wong AO et al (2008) Oxaliplatin plus irinotecan compared with irinotecan alone as second-line treatment after single-agent fluoropyrimidine therapy for metastatic colorectal carcinoma. J Clin Oncol 26:4544–4550
Kim GP, Sargent DJ, Mahoney MR et al (2009) Phase III noninferiority trial comparing irinotecan with oxaliplatin, fluorouracil, and leucovorin in patients with advanced colorectal carcinoma previously treated with fluorouracil: N9841. J Clin Oncol 27:2848–2854
Sobrero AF, Maurel J, Fehrenbacher L et al (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26:2311–2319
Langer C, Kopit J, Awad M et al (2008) Analysis of K-RAS mutations in patients with metastatic colorectal cancer receiving cetuximab in combination with irinotecan: results from the EPIC trial. In: 33rd European Society of Medical Oncology, Stockholm
Andre T, Blons H, Mabro M et al (2012) Panitumumab combined with irinotecan for patients with KRAS wild-type metastatic colorectal cancer refractory to standard chemotherapy: a GERCOR efficacy, tolerance, and translational molecular study. Ann Oncol [Epub ahead of print]
Seymour MT, Brown SR, Richman S et al (2011) Panitumumab in combination with irinotecan for chemoresistant advanced colorectal cancer: results of PICCOLO, a large randomised trial with prospective molecular stratification. Eur J Cancer 47(Suppl 1):6007
Lacouture ME, Mitchell EP, Piperdi B et al (2010) Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 28:1351–1357
Acknowledgments
The authors thank the patients and the medical and nursing staff of all the participating institutions: Also, the authors acknowledge Inmaculada Ruiz de Mena from TTD Data Center and Marta Llorens and Xavier Núñez from TFS Trial Form Support for monitoring, statistics and data management. This research, including data collection, analysis and medical writing services, was funded by Amgen. The authors also thank Dr. Beatriz Gil-Alberdi from HealthCo (Madrid, Spain) for her assistance in the preparation of the manuscript. Supported by the Spanish Cooperative Group for the Treatment of Digestive Tumors (TTD), Madrid, Spain.
Conflict of interest
Alfredo Carrato, consultant or advisory role (Amgen, Merck Serono) and research funding (Amgen). Fernando Rivera, consultant or advisory role (Amgen) and research funding (Amgen). Enrique Aranda, consultant or advisory role (Roche and Merck Serono). All remaining authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Study TTD-06-04; EudraCT: 2006-006363-24; clinicaltrials.gov identifier NCT00475293 registered on May 17, 2007.
On behalf of the Spanish Cooperative Group for the Treatment of Digestive Tumors (TTD).
Rights and permissions
About this article
Cite this article
Carrato, A., Gómez, A., Escudero, P. et al. Panitumumab and irinotecan every 3 weeks is an active and convenient regimen for second-line treatment of patients with wild-type K-RAS metastatic colorectal cancer. Clin Transl Oncol 15, 705–711 (2013). https://doi.org/10.1007/s12094-012-0993-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-012-0993-x