Abstract
Introduction
Sunitinib is a multiselective oral inhibitor of several tyrosine-kinase receptors that has demonstrated its efficacy in patients with metastatic and/or unresectable gastrointestinal stroma tumours (GIST) who were resistant to or intolerant to previous treatment with imatinib. The purpose of this study is to assess the cost-effectiveness of sunitinib vs. best supportive care (BSC) in GIST as a second-line treatment, from the perspective of the Spanish National Health System.
Materials and methods
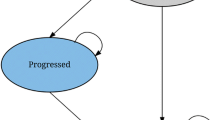
A Markov model was used to assess the cost effectiveness of sunitinib (50 mg/day, 4 weeks “on” and 2 weeks “off”) vs. BSC in GIST as a second-line treatment. Transition probabilities between the three health states considered in the model (progression-free survival (PFS), progression and death) were obtained from a clinical trial [Demetri et al. (2006) Lancet 368:1329–1338]. Health resource data (drugs, medical visits, laboratory and radiology tests, palliative care and adverse events) were obtained from an expert panel. Deterministic and probabilistic sensitivity analyses were conducted.
Results
Projected PFS years, life years (LY) and quality of life adjusted years (QALYs) were higher for sunitinib compared with BSC: 0.50 vs. 0.24, 1.59 vs. 0.88 and 1.00 vs. 0.55. Mean costs per patient were €23,259 with sunitinib and €1,622 with BSC. The incremental cost-effectiveness ratios (ICERs) obtained were: €4,090/month PFS, €30,242/LY and €49,090/QALY gained. The most influential variables for the results were the efficacy and unit cost of sunitinib.
Conclusions
According to the efficiency thresholds for oncology patients in developed countries, sunitinib is considered cost-effective vs. BSC with acceptable costs per LY and QALY gained.
Similar content being viewed by others
References
Muñoz C, Sabah S, Navarro A et al (2006) Tumores del estroma gastrointestinal: una revisión de la literatura. Gastr Latinoam 1:43–51
Goettsch WG, Bos SD, Breekveldt-Postma N et al (2005) Incidence of gastrointestinal stromal tumours is underestimated: results of a nation-wide study. Eur J Cancer 41:2868–2872
Nilsson B, Bumming P, Meis-Kindblom JM et al (2005) Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era; a population based study in western Sweden. Cancer 103:821–829
Mucciarini C, Rossi G, Bertolini F et al (2004) Gastrointestinal stromal tumors (GIST): Evaluation on malignancy and prognosis in 113 cases retrieved from a population based cancer registry of northern Italy. ASCO [Abstract 4232]
Ringenbach F, Viennet G, Monnien F et al (2005) Prevalence of GISTs in primary benign and malignant mesenchymal neoplasms of the gastrointestinal tract and the abdomen: preliminary pathological retrospective study of 180 cases from 1990 to 2000. ASCO GI Cancer Symp [Abstract 20]
Tryggvasson G, Gislason H, Magnusson M et al (2005) Gastrointestinal stromal tumors in Iceland, 1990–2003: The Icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer 117:289–293
Miettinen M, Lasota J (2003) Gastrointestinal stromal tumors (GISTs): definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol 54:3–24
Blanke CD, Eisemberg BL, Heinrich MC et al (2001) Gastrointestinal stromal tumors. Current Trat Options Oncol 6:1–5
De Pas T, Casali PG, Toma S et al (2003) Gastrointestinal stromal tumors: should they be treated with the same systemic chemotherapy as other soft tissue sarcomas?. Oncology 64:186–188
Garcia-del-Muro X, López-Pousa A, Buesa JM et al (2001) Temozolamide as a 6-week continuous oral Schedule in advanced sofá tissue sarcomas (STS): a phase II trial of the Spanish Group for Research on Sarcomas (GEIS). Proc Ann Meet Am Soc Clin Oncol 20:A354
Ryan DP, Puchalsky T, Supko JG et al (2002) A phase II and pharmacokinetics of ecteinascidin 743 in patients with gastrointestinal stromal tumors. Oncologist 7:531–538
Patel SR, Gandhi V, Jenkins J et al (2001) Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. J Clin Oncol 19:3483–3489
Corless CL, McGreevey L, Haley A et al (2002) KIT mutations are common in incidental gastrointestinal stromal tumors one centimetre or less in size. Am J Pathol 160:1567
Ng EH, Pollock RE, Munsell MF et al (1992) Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg 215:268
McGrath PC, Neifeld JP, Lawrence W et al (1987) Gastrointestinal sarcomas. Analysis of prognostic factors. Ann Surg 206:706
Rossi CR, Mocellin S, Mencarelli R et al (2003) Gastrointestinal stromal tumors: from a surgical to a molecular approach. Int J Cancer 107:171–176
Duffaud F, Blay JY (2003) Gastrointestinal stromal tumors: biology and treatment. Oncology 65:187–197
de Silva CM, Reid R (2003) Gastrointestinal stromal tumors (GIST): C-kit mutations, CD117 expression, differential diagnosis and targeted cancer therapy with imatinib. Pathol Oncol Res 9:13–19
Miettinen M, Lasota J (2003) Gastrointestinal stromal tumors (GISTs): definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol 54:3–24
Casper ES (2000) Gastrointestinal stromal tumors. Curr Treat Options Oncol 1:267–273
Demetri GD, Von Meheren M, Blanke CD et al (2002) Efficacy and safety of imesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472–480
Van Oosterom AT, Judson I, Verweij J et al (2001) Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumors: a phase I study. Lancet 358:1421–1423
Van Oosterom AT, Judson I, Verweij J et al (2002) Update of phase I study of imatinib (STI571) in advanced soft tissue sarcomas and gastrointestinal stromal tumors: a report of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 38: S83–S87
Benjamin RS, Rankin C, Fletcher C et al (2003) Phase III dose randomized study of imatinib mesylate (STI571) for GIST: intergroup S0033 early results (abstract 3271). Proc Am Soc Clin Oncol 22:814
Verweij J, Casali PG, Zalcberg J et al (2004) Progression-free-survival in gastrointestinal stromal tumors with high dose imatinib: randomized trial. Lancet 364:1127–1134
European Medicines Agency. Sutent European Public Assessment Report. Available at: http://www.emea.europa.eu/humandocs/Humans/EPAR/sutent/sutent.htm (published 17.12.07; accessed: 11.01.08)
Goodman VL, Rock EP, Dagher R et al (2007) Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res 13:1367–1373
Demetri GD, van Oosterom AT, Garrett CR et al (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368:1329–1338
Catálogo de Especialidades Farmacéuticas. Consejo General de Colegios Oficiales de Farmacéuticos. Available at: http://www.portalfarma.com (accessed: 23.03.07)
Oblikue Consulting. Base de Datos de Costes Sanitarios eSalud. Available at: http://www.oblikue.com/bddcostes/
Ojeda B, de Sande LM, Casado A et al (2003) Cost-minimisation analysis of pegylated liposomal doxorubicin hydrochloride versus topotecan in the treatment of patients with recurrent epithelial ovarian cancer in Spain. Br J Cancer 89:1002–1007
Wilson J, Connock M, Song F et al (2005) Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation. Health Technol Assess 25:33–45
Huse DM, von Mehren M, Lenhart G et al (2007) Cost effectiveness of imatinib mesylate in the treatment of advanced gastrointestinal stromal tumours. Clin Drug Investig 27:85–93
Chabot I, LeLorier J, Blackstein ME (2008) The challenge of conducting pharmacoeconomic evaluations in oncology using crossover trials: the example of sunitinib for gastrointestinal stromal tumour. Eur J Cancer 44:972–977
Demetri GD, Huang X, Garret CR et al. Novel statistical analysis of long term survival to account for cross-over in a phase III trial of sunitinib versus placebo in advanced GIST after imatinib failure. 2008 ASCO Annual Metting. [Abstract 10524]
Aguilar M, Fernández S, González P et al (2007) Pharmacoeconomic impact of the sunitinib adverse events (AEs) prophylaxis treatment in Spain. Drug Safety 30:960 [Abstract P.090]
Barbieri M, Drummond M, Willke R et al (2005) Variability of cost-effectiveness estimates for pharmaceuticals in Western Europe: lessons for inferring generalizability. Value Health 8:10–23
Rocchi A, Menon D, Verma S, Millar E (2007) The role of economic evidence in Canadian reimbursement decision-making: to lambda and beyond. Value Health 4:771–783
Nadler E, Eckert B, Neumann PJ (2006) Do oncologist believe new caner drugs offer good value? Oncologist 11:90–95
Grusenmeyer AP, Wong YN (2007) Interpreting the economic literature in oncology. J Clin Oncol 25:196–202
Drummond MF, Mason AR (2007) European perspective on the costs and cost-effectiveness of cancer therapies. J Clin Oncol 25:191–195
Raftery J (2006) Review of NICE’s recommendations, 1999–2005. Br Med J 332:1266–1268
Uyl-de-Groot CA (2006) Economic evaluation of cancer therapies: more and better studies will lead to better choices in cancer care. Eur J Cancer 42:2862–2866
Author information
Authors and Affiliations
Corresponding author
Additional information
Currently not working at Pfizer Spain
Rights and permissions
About this article
Cite this article
Paz-Ares, L., García del Muro, X., Grande, E. et al. Cost-effectiveness analysis of sunitinib in patients with metastatic and/or unresectable gastrointestinal stroma tumours (GIST) after progression or intolerance with imatinib. Clin Transl Oncol 10, 831–839 (2008). https://doi.org/10.1007/s12094-008-0297-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-008-0297-3