Abstract
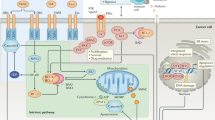
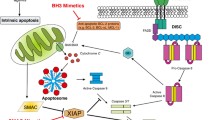
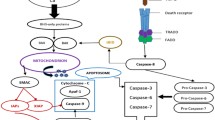
Chemotherapeutic agents and also radiotherapy trigger a series of signalling pathways in the cells that activate not only the apoptotic machinery but also cell survival pathways. Some of these pathways are also altered by genetic changes in specific type of tumours, and are different even between patients with the same tumour. Among these pathways, the majority of survival signals involve the ERK, AKT and nuclear factor-κB pathways and those related to cell death, which are driven mainly either by inhibition of such survival networks or by upregulation of the JNK/p38 MAP-kinases. Thus, the efficacy of a given chemotherapy appears as a result of the balance between cell death and survival pathways elicited in each individual tumour. Modulation of such survival pathways would help to increase the efficacy of chemotherapy. Different strategies based on conventional chemotherapy have been used in the past with modest success. The availability of new molecules such as inhibitors of survival pathways and the use of new technologies for the study of individual tumours would have a positive impact on patient survival.
Similar content being viewed by others
References
Nowell PC (1976) The clonal evolution of tumor cell populations. Science 194:23–28
Rajagopalan H, Lengauer C (2004) Aneuploidy and cancer. Nature 432:338–341
Longley DB, Johnston PG (2005) Molecular mechanisms of drug resistance. J. Pathol 205:275–292
Rickardson L, Fryknas M, Dhar S et al (2005) Identification of molecular mechanisms for cellular drug resistance by combining drug activity and gene expression profiles. Br J Cancer 93:483–492
Zhou BB, Elledge SJ (2000) The DNA damage response: putting checkpoints in perspective. Nature 408:433–439
Kruh GD (2003) Introduction to resistance to anticancer agents. Oncogene 22:7262–7264
Viatour P, Merville MP, Bours V, Chariot A (2005) Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci 30:43–52
Staal SP (1987) Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A 84:5034–5037
Jones PF, Jakubowicz T, Pitossi FJ et al (1991) Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci U S A 88:4171–4175
Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP (1998) Pten is essential for embryonic development and tumour suppression. Nat Genet 19:348–355
Testa JR, Bellacosa A (2001) AKT plays a central role in tumorigenesis. Proc Natl Acad Sci U SA 98:10983–10985
Bellacosa A, Kumar CC, Di Cristofano A, Testa JR (2005) Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res 94:29–86
Shawver LK, Slamon D, Ullrich A (2002) Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell 1:117–123
Liang K, Jin W, Knuefermann C et al (2003) Targeting the phosphatidylinositol 3-kinase/Akt pathway for enhancing breast cancer cells to radiotherapy. Mol Cancer Ther 2:353–360
Bianco R, Shin I, Ritter CA et al (2003) Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene 22:2812–2822
Hu L, Hofmann J, Lu Y et al (2002) Inhibition of phosphatidylinositol 3’-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res 62:1087–1092
Mansouri A, Zhang Q, Ridgway LD et al (2003) Cisplatin resistance in an ovarian carcinoma is associated with a defect in programmed cell death control through XIAP regulation. Oncol Res 13:399–404
Bonizzi G, Karin M (2004) The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25:280–288
Jobin C, Sartor RB (2000) The IkB/NF-B system. a key determinant of mucosa inflammation and protection. Am J Physiol Cell Physiol 278:C451–C562
Senftleben U, Cao Y, Xiao G et al (2001) Activation by IKK of a second, evolutionary conserved, NF-κB signaling pathway. Science 293:1495–1499
Romieu-Mourez R, Landesman-Bollag E, Seldin DC et al (2001) Roles of IKK kinases and protein kinase CK2 in activation of nuclear factor-kappaB in breast cancer. Cancer Res 61:3810–3818
Mathas S, Lietz A, Janz M et al (2003) Inhibition of NF-kappaB essentially contributes to arsenic-induced apoptosis. Blood 102:1028–1034
Bava SV, Puliappadamba VT, Deepti A et al (2005) Sensitization of taxol-induced apoptosis by curcumin involves down-regulation of nuclear factor-kappaB and the serine/threonine kinase Akt and is independent of tubulin polymerization. J Biol Chem 280:6301–6308
Li Y, Ahmed F, Ali S et al (2005) Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res 65:6934–6942
Mabuchi S, Ohmichi M, Nishio Y et al (2004) Inhibition of NFκB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem 279:23477–23485
Patel NM, Nozaki S, Shortle NH et al (2000) Paclitaxel sensitivity of breast cancer cells with constitutively active NF-κB is enhanced by IB super-repressor and parthenoide. Oncogene 19:4159–4169
Kim DW, Sovak MA, Zanieski G et al (2000) Activation of NF-kappaB/Rel occurs early during neoplastic transformation of mammary cells. Carcinogenesis 21:871–879
Ryan KM, Ernst MK, Rice NR, Vousden KH (2000) Role of NF-kappaB in p53-mediated programmed cell death. Nature 404:892–897
Nozaki S, Sledge GW Jr, Nakshatri H (2001) Repression of GADD153/CHOP by NF-kappaB: a possible cellular defense against endoplasmic reticulum stress-induced cell death. Oncogene 20:2178–2185
Costa C, Soares R, Reis-Filho JS et al (2002) Cyclo-oxygenase 2 expression is associated with angiogenesis and lymph node metastasis in human breast cancer. J Clin Pathol 55:429–434
Zhang S, Lin ZN, Yang CF et al (2004) Suppressed NF-kappaB and sustained JNK activation contribute to the sensitization effect of parthenolide to TNF-alpha-induced apoptosis in human cancer cells. Carcinogenesis 25:2191–2199
Weldon CB, Burow ME, Rolfe KW et al (2001) NF-kappa B-mediated chemoresistance in breast cancer cells. Surgery 130:143–150
Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103:239–252
Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410:37–40
Johnson GL, Lapadat R (2002) Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911–1912
Platanias LC (2003) Map kinase signaling pathways and hematologic malignancies. Blood 101:4667–4679
Bohmann D, Bos TJ, Admon A et al (1987) Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science 238:1386–1392
Sánchez I, Hughes RT, Mayer BJ et al (1994) Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature 372:794–798
Lin W, Kao HW, Robinson D et al (2000) Tyrosine kinases and gastric cancer. Oncogene 19:5680–5689
Wu CW, Li AF, Chi CW et al (2000) Human gastric cancer kinase profile and prognostic significance of MKK4 kinase. Am J Pathol 156:2007–2015
Sánchez-Pérez I, Murguia JR, Perona R (1998) Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene 16:533–540
Stadheim TA, Kucera GL (2002) c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for mitoxantrone-and anisomycin-induced apoptosis in HL-60 cells. Leuk Res 26:55–65
Teng DH, Perry WL 3rd, Hogan JK et al (1997) Human mitogen-activated protein kinase 4 as a candidate tumor suppressor. Cancer Res 57:4177–4182
Su GH, Song JJ, Repasky EA et al (2002) Mutation rate of MAP2K4/MKK4 in breast carcinoma. Hum Mutat 19:81
Wada T, Joza N, Cheng HY et al (2004) MKK7 couples stress signalling to G2/M cell-cycle progression and cellular senescence. Nat Cell Biol 6:215–226
Sánchez-Pérez I, Murguia JR, Perona R (1998) Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene 16:533–540
Sánchez-Pérez I, Perona R (1999) Lack of c-Jun activity increases survival to cisplatin. FEBS Lett 453:151–158
Sánchez-Pérez I, Martínez-Gomariz M, Williams D et al (2000) CL100/MKP-1 modulates JNK activation and apoptosis in response to cisplatin. Oncogene 19:5142–5152
Sánchez-Pérez I, Benitah SA, Martínez-Gomariz M et al (2002) Cell stress and MEKK1-mediated c-Jun activation modulate NFkappaB activity and cell viability. Mol Biol Cell 13:2933–2945
Wang HY, Cheng Z, Malbon CC (2003) Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett 191:229–237
Denkert C, Schmitt WD, Berger S et al (2002) Expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) in primary human ovarian carcinoma. Int J Cancer 102:507–51
Chattopadhyay S, Machado-Pinilla R, Manguan-García C, et al (2006) MKP1/CL100 controls tumor growth and sensitivity to cisplatin in non-small cell lung cancer 25:3335–3345
Author information
Authors and Affiliations
Additional information
Supported by an unrestricted educational grant from Pfizer.
Rights and permissions
About this article
Cite this article
Perona, R., Sánchez-Pérez, I. Signalling pathways involved in clinical responses to chemotherapy. Clin Transl Oncol 9, 625–633 (2007). https://doi.org/10.1007/s12094-007-0115-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-007-0115-3