Abstract
The metacommunity concept has proved to be a valuable tool for studying how space can affect the properties and assembly of competitive communities. However, the concept has not been as extensively applied to the study of food webs or trophically structured communities. Here, we demonstrate how to develop a modelling framework that permits food webs to be considered from a spatial perspective. We do this by broadening the classic metapopulation patch-dynamic framework so that it can also account for trophic interactions between many species and patches. Unlike previous metacommunity models, we argue that this requires a system of equations to track the changing patch occupancy of the various species interactions, not the patch occupancy of individual species. We then suggest how this general theoretical framework can be used to study complex and spatially extended food web metacommunities.




Similar content being viewed by others
References
Allesina S, Pascual M (2008) Network structure, predator–prey modules, and stability in large food webs. Theor Ecol 1:55–64
Amarasekare P (2008) Spatial dynamics of keystone predation. J Anim Ecol 77:1306–1315
Bascompte J, Sole RV (1998) Effects of habitat destruction in a prey–predator metapopulation model. J Theor Biol 195:383–393
Brose U, Pavao-Zuckerman M, Eklof ABJ, Berg MP, Cousins SH, Mulder C, Verhoef HA, Wolters V (2005) Spatial aspects of food webs. In: de Ruiter P, Wolters V, Moore JC (eds) Dynamic food webs: multispecies assemblages, ecosystem development and environmental change. Academic, Burlington, pp 463–469
Brose U, Williams RJ, Martinez ND (2006) Allometric scaling enhances stability in complex food webs. Ecol Lett 9:1228–1236
Calcagno V, Mouquet N, Jarne P, David P (2006) Coexistence in a metacommunity: the competition–colonization trade-off is not dead. Ecol Lett 9:897–907
Cottenie K, De Meester L (2003) Connectivity and cladoceran species richness in a metacommunity of shallow lakes. Freshw Biol 48:823–832
Cottenie K, De Meester L (2004) Metacommunity structure: synergy of biotic interactions as selective agents and dispersal as fuel. Ecology 85:114–119
Cottenie K, Michels E, Nuytten N, De Meester L (2003) Zooplankton metacommunity structure: regional vs. local processes in highly interconnected ponds. Ecology 84:991–1000
Crowley PH (1979) Predator-mediated coexistence—equilibrium interpretation. J Theor Biol 80:129–144
Gardner MR, Ashby WR (1970) Connectance of large dynamic (Cybernetic) systems—critical values for stability. Nature 228:784
Gouhier T, Guichard F, Gonzalez A (2010) Synchrony and stability of food webs in metacommunities. Am Nat (in press)
Guichard F (2005) Interaction strength and extinction risk in a metacommunity. Proc R Soc Lond B Biol Sci 272:1571–1576
Hastings A (1980) Disturbance, coexistence, history, and competition for space. Theor Popul Biol 18:363–373
Holt RD (1997) Community modules. In: Gange AC, Brown VK (eds) Multitrophic interactions in terrestrial ecosystems. Blackwell Science, Oxford, pp 333–349
Holt RD (2002) Food webs in space: on the interplay of dynamic instability and spatial processes. Ecol Res 17:261–273
Holyoak M, Leibold MA, Holt RD (eds) (2005) Metacommunities: spatial dynamics and ecological communities. University of Chicago Press, Chicago
Huffaker CB (1958) Experimental studies on predation: dispersion factors and predator-prey oscillations. Hilgardia 27:343–383
Keeling MJ (2002) Using individual-based simulations to test the Levins metapopulation paradigm. J Anim Ecol 71:270–279
Kneitel JM, Miller TE (2003) Dispersal rates affect species composition in metacommunities of Sarracenia purpurea inquilines. Am Nat 162:165–171
Koelle K, Vandermeer J (2005) Dispersal-induced desynchronization: from metapopulations to metacommunities. Ecol Lett 8:167–175
Lawton JH, Warren PH (1988) Static and dynamic explanations for patterns in food webs. Trends Ecol Evol 3:242–245
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613
Levins R (1969) Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Soc Am 15:237–240
Levins R (1970) Complex systems. In: Waddington ECH (ed) Towards a theoretical biology. Edinburgh University Press, Edinburgh, pp 73–88
Levins R, Culver D (1971) Regional coexistence of species and competition between rare species (mathematical model/habitable patches). Proc Natl Acad Sci U S A 68:1246
Loreau M, Downing A, Emmerson M, Gonzalez A, Hughes J, Inchausti P, Joshi J, Norberg J, Sala O (2002) A new look at the relationship between diversity and stability. In: Loreau M, Naeem S, Inchausti P (eds) Biodiversity and ecosystem functioning: synthesis and perspectives. Oxford University Press, Oxford, pp 79–91
Loreau M, Mouquet N, Gonzalez A (2003) Biodiversity as spatial insurance in heterogeneous landscapes. Proc Natl Acad Sci U S A 100:12765–12770
May RM (1972) Will a large complex system be stable. Nature 238:413
May RM (1973) Stability and complexity in model ecosystems. Princeton University Press, Princeton
May RM (1994) The effects of spatial scale on ecological questions and answers. In: Edwards PJ, May RM, Webb RN (eds) Large-scale ecology and conservation biology. Blackwell Scientific Publications, Oxford, pp 1–17
May RM, Nowak MA (1994) Superinfection, metapopulation dynamics, and the evolution of diversity. J Theor Biol 170:95–114
McCann K, Hastings A, Huxel GR (1998) Weak trophic interactions and the balance of nature. Nature 395:794–798
McCann KS, Rasmussen JB, Umbanhowar J (2005) The dynamics of spatially coupled food webs. Ecol Lett 8:513–523
Melian CJ, Bascompte J (2002) Food web structure and habitat loss. Ecol Lett 5:37–46
Michalski J, Arditi R (1995) Food-web structure at equilibrium and far from it—is it the same. Proc R Soc Lond B Biol Sci 259:217–222
Miller TE, Kneitel JM, Burns JH (2002) Effect of community structure on invasion success and rate. Ecology 83:898–905
Mouquet N, Loreau M (2002) Coexistence in metacommunities: the regional similarity hypothesis. Am Nat 159:420–426
Mouquet N, Loreau M (2003) Community patterns in source-sink metacommunities. Am Nat 162:544–557
Neutel AM, Heesterbeek JAP, van de Koppel J, Hoenderboom G, Vos A, Kaldeway C, Berendse F, de Ruiter PC (2007) Reconciling complexity with stability in naturally assembling food webs. Nature 449:599-U11
Otto SB, Rall BC, Brose U (2007) Allometric degree distributions facilitate food-web stability. Nature 450:1226-U7
Paine RT (1988) Food webs—road maps of interactions or grist for theoretical development. Ecology 69:1648–1654
Pimm SL (1984) The complexity and stability of ecosystems. Nature 307:321–326
Pimm SL, Lawton JH (1978) Feeding on more than one trophic level. Nature 275:542–544
Polis GA (1991) Complex trophic interactions in deserts— an empirical critique of food-web theory. Am Nat 138:123–155
Rooney N, McCann K, Gellner G, Moore JC (2006) Structural asymmetry and the stability of diverse food webs. Nature 442:265–269
Ryall KL, Fahrig L (2006) Response of predators to loss and fragmentation of prey habitat: a review of theory. Ecology 87:1086–1093
Schoenly K, Cohen JE (1991) Temporal variation in food web structure—16 empirical cases. Ecol Monogr 61:267–298
Swihart RK, Feng ZL, Slade NA, Mason DM, Gehring TM (2001) Effects of habitat destruction and resource supplementation in a predator–prey metapopulation model. J Theor Biol 210:287–303
Tilman D (1994) Competition and biodiversity in spatially structured habitats. Ecology 75:2–16
Van der Gucht K, Cottenie K, Muylaert K, Vloemans N, Cousin S, Declerck S, Jeppesen E, Conde-Porcuna JM, Schwenk K, Zwart G, Degans H, Vyverman W, De Meester L (2007) The power of species sorting: local factors drive bacterial community composition over a wide range of spatial scales. Proc Natl Acad Sci U S A 104:20404–20409
van Nouhuys S, Hanski I (2005) Metacommunities of butterflies, their host plants, and their parasitoids. In: Holyoak M, Leibold MA, Holt RD (eds) Metacommunities: spatial dynamics and ecological communities. University of Chicago Press, Chicago, pp 99–121
Vandermeer J (1973) Regional stabilization of locally unstable predator-prey relationships. J Theor Biol 41:161–170
Warren PH (1989) Spatial and temporal variation in the structure of a fresh-water food web. Oikos 55:299–311
Zeigler BP (1977) Persistence and patchiness of predator–prey systems induced by discrete event population exchange mechanisms. J Theor Biol 67:687–713
Acknowledgements
We would like to thank M. Cherif, S. Leroux, Z. Long, C. de Mazancourt and F. Guichard for useful comments and discussions. A. Gonzalez and M. Loreau are supported by the Canada Research Chair program, NSERC Discovery grants, and an FQRNT team grant.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A
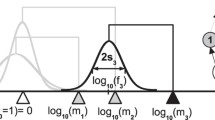
We can appreciate the motivation underlying Eq. 4 by considering the portion of a hypothetical food web shown in Fig. 5. The subgraph S 4,9 of the food web graph in Fig. 5 represents all the species and feeding links lying on a directed path between species 4 and species 9 (solid black lines). From Eq. 3, we know that f (S 4,9) gives the fraction of patches occupied by species 9 that are also occupied by species 4. In this case
However, what we are actually interested in is the fraction of species 9-occupied patches where species 9 will be affected by extinctions of species 4. Looking at Fig. 5, we see that extinction of species 4 in patches containing the food chain sequence 4, 5, 7, 8, and 9 will not lead to the extinction of species 9, since an intermediate species 7 can switch predation to 4’s resource species 3, as indicated by the (3, 7) link (dashed line). As a result, a patch with the food chain sequence 3, 4, 5, 7, 8 and 9 will reassemble, after 4 goes extinct, into 3, 7, 8 and 9.
In order to know how the regional abundance of a species i is affected by the extinction rate of any particular species k below it, we need to know what fraction of patches containing species i also contains some intermediate species m capable of switching consumption to one of k’s resources upon k’s extinction. This is given by the second term in Eq. 4, \( f\left( {\bigcup\limits_{m \in C(l)} {\left( {{S_{k,m}} \cup {S_{m,i}}} \right)} } \right) \). For the example given here,
Subtracting Eq. 8 from Eq. 7, as per Eq. 4 gives us the net fraction of patches occupied by 9, where 9 will go extinct upon 4 going extinct whenever 4 is consuming resource 3: \( \Phi_{4,9}^3 = \left( {{\rho_{\left( {4,5} \right)}} \cdot {\rho_{\left( {5,6} \right)}} \cdot {\rho_{\left( {6,8} \right)}} \cdot {\rho_{\left( {8,9} \right)}}} \right) + {\rho_{\left( {4,9} \right)}} \).
Appendix B
We demonstrate here the application of Eq. 2 for the top two pairwise interactions in Fig. 2c (we assume v 5,4 > v 6,4 and v 3,1 > v 6,1).
For the (6, 7) interaction, the first term in Eq. 4 becomes \( A = {c_{7,6}}{p_{\left( {6,7} \right)}}\left( {{p_{\left( {1,6} \right)}} + {p_{\left( {4,6} \right)}} - {p_{\left( {6,7} \right)}}} \right) \). Here, the growth in the fractions of patches with the (6, 7) interactions is due to the total number of species 7 colonisers (c 7,6 p (6,7)) landing on available resource 6 patches, itself given by the total fractions of resource 6 patches (p (1,6) + p (4,6)), minus those already occupied by consumer 7 (p (6,7)).
The second term given by \( B = \left( {{e_{7,6}} + {\mu_{7,6}}} \right){p_{\left( {6,7} \right)}} \) simply gives the extinction rate of the (6, 7) interaction due to the extinction rate of consumer 7 (e 7,6) and the rate that resource 6 is driven extinct due to predation by consumer 7, (μ 7,6).
Since there are no competitors for resource 6 and no alternative resources for consumer 7 aside from 6, terms C, D and E are all equal to 0. However, a decrease in the (6, 7) interaction can occur due to extinctions of species further down the food web. The effect that species extinctions further down the food web has on the (6, 7) interaction will be determined by the degree of patch overlap between the (6, 7) interaction and the rate of extinction for each species below 6. In the F term, the extinction rates of all species interactions below (6, 7), multiplied by the fraction of the 7-occupied patches that will be affected by that extinction are summed (see Appendix A for an example). When this sum is multiplied by the density of (6, 7) patches, p (6,7), we get \( F = {p_{\left( {6,7} \right)}}\left( {\left( {{e_{6,1}}{\rho_{\left( {1,6} \right)}} + {\rho_{\left( {4,6} \right)}}} \right.\left( {\left( {{e_{6,4}} + {\mu_{6,4}}} \right) + \left( {{e_{4,3}} + {\mu_{4,3}}} \right) + {\rho_{\left( {2,3} \right)}}\left( {{e_{3,2}} + {\mu_{3,2}}} \right) + {\rho_{\left( {1,3} \right)}}\left( {{e_{3,1}} + {\mu_{3,1}}} \right)} \right)} \right) \).
The (6, 7) interaction can also decrease due to competitive displacement of species further down the food web—in this case, species 5 displacing 6 from resource 4 patches. Thus, here, G is equal to the number of species 5 colonisers, c 5,4 p (4,5), that successfully land on and displace consumer species 6 from resource 4 in food chains with the (6, 7) interaction, ρ (6,4) p (6,7), giving us G = p (6,7) (c 5,4 p (4,5) ρ (6,4)). Finally, H = 0, since there is no predator species that can drive 7 extinct from top–down effects. The overall equation for the (6, 7) interaction is then
Similarly for the (4, 6) interaction, A gives growth of the interaction due to colonisation of available resource 4 patches by consumer 6. However, now, in order to determine the total number of species 6 colonisers produced, one must sum over all the resource patches occupied by 6 (c 6,4 p (4,6) + c 6,1 p (1,6)), giving us \( A = \left( {{c_{6,4}}{p_{\left( {4,6} \right)}} + {c_{6,1}}{p_{\left( {1,6} \right)}}} \right)\left( {{p_{\left( {3,4} \right)}} - {p_{\left( {4,5} \right)}} - {p_{\left( {4,6} \right)}}} \right) \). B is defined similarly to the previous example.
The (4, 6) interaction, unlike (6, 7), can decrease due to competitive displacement of the consumer species; in this case, 6 can be displaced from resource 4 by 5 giving us C = c 5,4 p (4,5) (p (4,6)). Since there is no way 6 can directly switch from some alternative resource onto 4, both D and E are equal to 0. The F and H terms are determined similarly to the previous example, while G = 0, since there are no species below the interaction that can be displaced by superior competitors. The overall equation then for the (4, 6) interaction is
Appendix C
Algorithm for transforming a food web graph
Below, we outline an algorithm that can transform the food web graph so that no directed path in the graph will represent an a priori impossible food chain configuration. The algorithm required is relatively straightforward: (1) move up the vertex set until you come to a generalist species, t, with more than one incoming edge, at least one of which is a bypass link, which directly connects the consumer to a resource further down one of its other food chains. (2) Start at the resource vertex in one of the bypass links that has the lowest index value, and from there, start moving up the vertex set one index number at a time, checking all the incoming edges for each vertex while doing so. If the current vertex, k, has more than one incoming edge, at least one of which is from a directed path rooted in one of the bypass resources and at least one of which is from a directed path not rooted in one of the bypass resources, and if at least one of vertex k’s outgoing edges is on a directed path towards t, then split vertex k into two parallel vertices, each with the same outgoing edge as before, but no incoming edges. (3) Attach to the original vertex, k, any incoming edges from directed paths that were not rooted in one of the bypass resources, and to the new vertex, k’, attach the incoming edges from paths that are rooted in the one of the bypass resources. (4) Continue this until you arrive at the consumer vertex t and then disconnect all incoming edges from t that are part of a directed path from one of the bypass species. (5) Start again at (1) moving up the food web looking for the next generalist predator with bypass loops in order to establish a new t, and then repeat (2)–(4).
This algorithm can be applied to the network before the system of differential equations is defined. A simple example of transformation can be observed for the graph in Fig. 2c, which becomes, by the above method, the graph shown in Fig. 6. In Fig. 6, the distribution of each consumer’s feeding links among its prey can be considered independently of how the prey’s own feeding links are distributed among its own resources and so on. As a result, the frequency of patch overlap between any two species in a directed path or food chain can be determined by multiplying the ρ values of the pairwise interactions along the path between them.
The food web in Fig. 2c after being transformed by the algorithm described in Appendix C to ensure that all directed paths depicted in the food web graph are not a priori impossible food chains
Rights and permissions
About this article
Cite this article
Pillai, P., Loreau, M. & Gonzalez, A. A patch-dynamic framework for food web metacommunities. Theor Ecol 3, 223–237 (2010). https://doi.org/10.1007/s12080-009-0065-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-009-0065-1