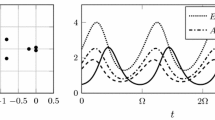
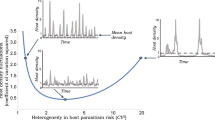
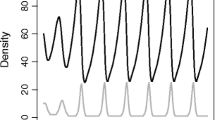
Abstract
There are many well-documented cases in which multiple parasitoids can coexist on a single host species. We examine a theoretical framework to assess whether parasitoid coexistence can be explained through differences in timing of parasitoid oviposition and parasitoid emergence. This study explicitly includes the phenology of host and parasitoid development and explores how this mechanism affects the population dynamics. Coexistence of the host with two parasitoids requires a balance between parasitoid fecundity and survival and occurs most readily if one parasitoid attacks earlier but emerges later than the other parasitoid. The host density can either be decreased or increased when a second coexisting parasitoid is introduced into the system. However, there always exists a single parasitoid type that is most effective at depressing the host density, although this type may not be successful due to parasitoid competition. The coexistence of multiple parasitoids also affects the population dynamics. For instance, population oscillations can be removed by the introduction of a second parasitoid. In general, subtle differences in parasitoid phenology can give rise to different outcomes in a host–multi-parasitoid system, and this may offer some insight into why establishing criteria for the ‘ideal’ biological control agent has been so challenging.





Similar content being viewed by others
References
Amarasekare P (2002) Interference competition and species coexistence. Proc R Soc Lond B 269(1509):2541–2550
Amarasekare P (2003) Diversity-stability relationships in multitrophic systems: an empirical exploration. J Anim Ecol 72(5):713–724
Amarasekare P (2007) Trade-offs, temporal variation, and species coexistence in communities with intraguild predation. Ecology 88(11):2720–2728
Beddington JR, Free CA, Lawton JH (1975) Dynamic complexity in predator-prey models framed in difference equations. Nature 255:58–60
Bonsall MB, Hassell MP, Asefa G (2002) Ecological trade-offs, resource partitioning, and coexistence in a host-parasitoid assemblage. Ecology 83(4):925–934
Borer ET, Briggs C, Murdoch WW, Swarbrick S (2003) Testing intraguild predation theory in a field system: does numerical dominance shift along gradients of productivity? Ecol Lett 6:929–935
Briggs CJ, Nisbet RM, Murdoch WW (1993) Coexistence of competing parasitoid species on a host with a variable life cycle. Theor Popul Biol 44:341–373
Chesson PL, Case TJ (1986) Overview: nonequilibrium community theories chance variability history and coexistence. In: Diamond J, Case TJ (eds) Community ecology. Harper and Row, Philadelphia
Cobbold CA, Lewis MA, Lutscher F, Roland J (2005) How parasitism affects critical patch-size in a host-parasitoid model: application to the forest tent caterpillar. Theor Popul Biol 67:109–125
Comins HN, Hassell MP (1996) Persistence of multispecies host-parasitoid interactions in spatially distributed models with local dispersal. J Theor Biol 183(1):19–28
Godfray HCJ, Hassell MP, Holt, RD (1994) The population dynamic consequences of phenological asynchrony between parasitoids and their hosts. J Anim Ecol 63:1–10
Hawkins BA (1994) Pattern and process in host-parasitoid interactions. Cambridge University Press, Cambridge
Hawkins BA, Thomas MB, Hochberg ME (1993) Refuge theory and biological control. Science, New Series 262(5138):1429–1432
Hochberg ME (1996) Consequences for host population levels of increasing natural enemy species richness in classical biological control. Am Nat 147(2):307–318
Hochberg ME, Hawkins BA (1992) Refuge as a predictor of parasitoid diversity. Science, New Series 255(5047):973–976
Hochberg ME, Hawkins BA (1993) Predicting parasitoid species richness. Am Nat 142(4):671–693
Hochberg ME, Holt RD (1995) Refuge evolution and the population-dynamics of coupled host-parasitoid associations. Evol Ecol 9(6):633–661
Hogarth WL, Diamond P (1984) Interspecific competition in larvae between entomophagous parasitoids. Am Nat 124(4):552–560
Klopfer ED, Ives AR (1997) Aggregation and the coexistence of competing parasitoid species. Theor Popul Biol 52(3):167–178
MacArthur RH, Levins R (1967) The limiting similarity, convergence and divergence of coexisting species. Am Nat 101:377–385
May RM, Hassell MP (1981) The dynamics of multiparasitoid-host interactions. Am Nat 117(3):234–261
Memmott J, Godfray HCJ, Gauld ID (1994) The structure of a tropical host parasitoid community. J Anim Ecol 63(3):521–540
Metz JAJ, Nisbet RM, Geritz SAH (1992) How should we define “fitness” for general ecological scenarios? TREE 7:198–202
Murdoch WW, Briggs CJ, Nisbet RM (1997) Dynamical effects of host size- and parasitoid state-dependent attacks by parasitoids. J Anim Ecol 66(4):542–556
Nicholson AJ, Bailey VA (1935) The balance of animal populations. Part I. Proc Zool Soc Lond 3:551–598
Parry D (1994) The impact of predators and parasitoids on natural and experimentally created populations of forest tent caterpillar, Malacosoma disstria Hübner (lepidoptera: lasiocampidae). Master’s thesis, University of Alberta, Edmonton, Alberta
Parry D (1995) Larval and pupal parasitism of forest tent caterpillar, Malacosoma disstria Hübner (lepidoptera: lasiocampidae), in Alberta Canada. Can Entomol 127:877–893
Pederson BS, Mills NJ (2004) Single vs multiple introduction in biological control: the roles of parasitoid efficiency, antagonism and niche overlap. J Appl Ecol 41:973–984
Plantard O, Rasplus JY, Hochberg ME (1996) Resource partitioning in the parasitoid assemblage of the oak galler Neuroterus quercusbaccarum L (Hymenoptera: Cynipidae). Oecologica 17(1):1–15
Porter EE, Hawkins BA (2003a) Coexistence of specialist parasitoids with host refuges in the laboratory and the dynamics of spatial heterogenity in attack rate. OIKOS 100:232–240
Porter EE, Hawkins BA (2003b) The influence of varying spatial heterogenity on the refuge model for coexistence of specialist parasitoid assemblages. OIKOS 100:241–250
Roland J (1994) After the decline: what maintains low winter moth density after successful biological control? J Anim Ecol 63:392–398
Snyder RE, Borer ET, Chesson P (2005) Examining the relative importance of spatial and nonspatial coexistence mechanisms. Am Nat 166(4):E75–E94
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A: Deriving the host-multi-parasitoid model
In this section, a continuum analogy, involving a system of ordinary differential equations, is used to show how the model in Section ‘Host–multiple-parasitoid model’ for two parasitoids, P and Q, and one host, H, is obtained. For concreteness, we assume that the order of attack is exactly as shown in Fig. 1, and that density–dependence acts for the whole season, i.e. T dd = 1. Other arrangements of attack window and choices of T dd can be applied in a similar way.
The equations for the continuum model are
Here, H n , P n and Q n are constants which correspond to the density of hosts and adult parasitoids of type (P, Q) at the start of the season, which we take to be the density in year n. It is important to make clear that by P(t) and Q(t) we actually mean hosts parasitised by parasitoid P or Q. On the day the parasitoid emerges, the host is killed, so the parasitoid density instantaneously becomes equal to the parasitised host density. The equations above govern the most general situation when both P and Q are attacking the host. For periods where one (or both) of the parasitoids is not attacking, we should set the associated searching efficiency to zero. The host mortality term is due to intraspecific competition for resources. We assume that parasitised hosts compete in the same way as unparasitised hosts. Mortality depends on the density of hosts at the start of the season. One interpretation of this term is that hosts have reduced fitness if the parent generation was at a high density and experienced strong competition.
Solving Eq. 9 for H(t), and then substituting into the equations for P(t) and Q(t), gives the following general solutions:
where c 1, c 2, c 3 are integration constants. We now apply the boundary conditions in each segment of the year indicated in Fig. 1 to work out H(n + 1), P(n + 1), Q(n + 1), i.e. the densities at the start of year n + 1.
First, we consider the period 0 < t < t ps . The initial host density is H(0) = H n . Since no parasitoid attacks the host in this period, we should set a p = a q = 0 in Eqs. 12–14 and the boundary conditions are P(0) = P(t ps ) = 0 and Q(0) = Q(t ps ) = 0. Therefore, at the end of the first period, we obtain
These will be initial conditions for the solutions in the next time period.
We now consider round 1, namely, t ps < t < t qs . Since Q has not begun its attack, we set a q = 0 in Eqs. 12–14. Our initial conditions are Eq. 15, which allow us to determine the constants as follows:
Therefore, at the end of round 1, we have densities
These give the initial conditions for round 2.
In round 2, i.e. t qs < t < t pf , both P and Q are attacking. Using the initial conditions Eq. 16 at time t qs in the solutions Eqs. 12–14, we find
So, using these values for the constants, at the end of round 2, we have
which become the initial conditions for the next round.
In round 3, t pf < t < t qf , P has stopped attacking, so we should set a p = 0 in Eqs. 12–14. Using Eq. 17 as initial conditions, we obtain
So by the end of round 3, the densities are
For t > t qf , both parasitoids have finished attacking (ovipositing on) the hosts. Density-dependence continues to affect the parasitised host until the parasitoids emerge from the host at α p , α q . Unparasitised hosts continue to experience density-dependence for the remainder of the season. Therefore, the densities at t = n + 1 are
To obtain the model in Section ‘Host–multiple-parasitoid model’, we assume that, just prior to the start of the next season, adult hosts reproduce with, on average, e r offspring per host, which means we multiply the equation for H by e r. In a more general setting where the density–dependent phase has length T dd , we can obtain the corresponding model by scaling g →g T dd . (Note that in this model framework, density dependence always appears in the equations as an overall multiplicative factor.)
Appendix B: Host–N-parasitoid model for equal attack windows
In the special case when all attack windows are equal, the equations for N parasitoids, P (i), are described by the following equations:
where t s , t f are the start and finish times of the attack window and i = 1, ..., N. Each parasitoid species P (i) has an associated searching efficiency, a i , and fraction of host density-dependence which it experiences, α i .
Rights and permissions
About this article
Cite this article
Hackett-Jones, E., Cobbold, C. & White, A. Coexistence of multiple parasitoids on a single host due to differences in parasitoid phenology. Theor Ecol 2, 19–31 (2009). https://doi.org/10.1007/s12080-008-0025-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-008-0025-1