Abstract
Cell adhesion and migration are complex processes that require integrin activation, the formation and dissolution of focal adhesion (FAs), and linkage of actin cytoskeleton to the FAs. The IPP (ILK, PINCH, Parvin) complex regulates FA formation via binding of the adaptor protein ILK to β1 integrin, PINCH and parvin. The signaling protein Rsu1 is linked to the complex via binding PINCH1. The role of Rsu1 and PINCH1 in adhesion and migration was examined in non-transformed mammary epithelial cells. Confocal microscopy revealed that the depletion of either Rsu1 or PINCH1 by siRNA in MCF10A cells decreased the number of focal adhesions and altered the distribution and localization of β1 integrin, vinculin, talin and paxillin without affecting the levels of FA protein expression. This correlated with reduced adhesion, failure to spread or migrate in response to EGF and a loss of actin stress fibers and caveolae. In addition, constitutive phosphorylation of actin regulatory proteins occurred in the absence of PINCH1. The depletion of Rsu1 caused significant reduction in PINCH1 implying that Rsu1 may function by regulating levels of PINCH1. However, while both Rsu1- or PINCH1-depleted cells retained the ability to activate adhesion signaling in response to EGF stimulation, only Rsu1 was required for EGF-induced p38 Map Kinase phosphorylation and ATF2 activation, suggesting an Rsu1 function independent from the IPP complex. Reconstitution of Rsu1-depleted cells with an Rsu1 mutant that does not bind to PINCH1 failed to restore FAs or migration but did promote spreading and constitutive p38 activation. These data show that Rsu1-PINCH1 association with ILK and the IPP complex is required for regulation of adhesion and migration but that Rsu1 has a critical role in linking integrin-induced adhesion to activation of p38 Map kinase signaling and cell spreading. Moreover, it suggests that Rsu1 may regulate p38 signaling from the IPP complex affecting other functions including survival.
Similar content being viewed by others
Introduction
The regulation of cell adhesion and migration is an essential feature of numerous biological processes including embryonic development, wound healing, and tumor metastasis. These processes rely on the formation and dissolution of integrin based adhesive complexes and controlled actomyosin contractility. The IPP complex, centrally composed of integrin linked-kinase (ILK), the adaptor protein PINCH and parvin, is an organizing and signaling network for integrin adhesion. The composition and the functions of the complex have been the subjects of recent reviews (Stanchi et al. 2009; Cabodi et al. 2010; Wickstrom et al. 2010b; Qin and Wu 2012). IPP functions in integrin activation, actin cytoskeleton remodeling (Sakai et al. 2003; Li et al. 2005) and FA formation (Sakai et al. 2003; Stanchi et al. 2009). The Ras suppressor protein 1 (Rsu1) is a leucine rich repeat protein that has a role in the IPP complex activity via binding to the LIM 5 domain of the adaptor protein PINCH1 (Kadrmas et al. 2004; Dougherty et al. 2005). Rsu1 co-localizes with PINCH1 at sites of focal adhesions in mammalian cells and muscle cell attachment in Drosophila (Kadrmas et al. 2004; Dougherty et al. 2005; Dougherty et al. 2008; Montanez et al. 2012). The inhibition of PINCH-ILK or PINCH-Rsu1 interaction results in decreased cell spreading and reduced motility of mammalian cells (Tu et al. 1999; Zhang et al. 2002a, b; Dougherty et al. 2005) such that Rsu1 and PINCH1 are independently required for migration in the mammary epithelial cell line MCF10A (Simpson et al. 2008).
The regulatory function of PINCH in adhesion, spreading and migration depends on its association with multiple accessory proteins (Li et al. 2005; Liang et al. 2005; Xu et al. 2005). PINCH1 and 2 consist of five LIM domains and a carboxyl terminal nuclear localization signal (NLS) (Hobert et al. 1999; Braun et al. 2003) and both bind to an ILK ankyrin repeat through their N terminal LIM1 domain (Li et al. 1999; Zhang et al. 2002a, b). The IPP complex connects integrins to growth factor signaling through the binding of LIM4 of PINCH1 to Nck2 (Tu et al. 1998; Velyvis et al. 2003). Thymosin β4, which maintains pools of actin monomers in cells, binds to the LIM 4 and LIM 5 domains (Bock-Marquette et al. 2004). In addition to Rsu1, the LIM5 domain of PINCH1 has also been reported to bind the protein phosphatase-1α (PP1α) (Eke et al. 2010). Beyond PINCH1 interaction, ILK associates with the actin cytoskeleton through the binding of α parvin (Tu et al. 2001) and binding to the LIM-domain adaptor protein paxillin (Nikolopoulos and Turner 2000). Parvins regulate actin cytoskeleton dynamics and FA turnover by the association with a variety of molecules including paxillin, actin regulatory protein kinases (TESK1) (LaLonde et al. 2005), actin crosslinking proteins (α-actinin) (Yamaji et al. 2004), and guanine nucleotide exchange factor α-PIX (Rosenberger et al. 2003). ILK links the complex to integrins via binding of its C-terminal domain to β subunits of integrins.
The phenotypes observed upon Rsu1 expression support a role for Rsu1 in IPP function. Ectopic expression of Rsu1 in NIH 3 T3 mouse fibroblasts increased cell spreading and the accumulation of actin while its depletion resulted in a decrease in cell adhesion in mammalian cells and decreased integrin-dependent functions in Drosophila (Masuelli and Cutler 1996; Kadrmas et al. 2004; Dougherty et al. 2005). The ectopic expression of Rsu1 cDNA also prevented Ras transformation and inhibited anchorage independent growth in human tumor cell lines (Cutler et al. 1992; Tsuda et al. 1995; Vasaturo et al. 2000). Changes in the level of Rsu1 correlate with altered JNK activity (Masuelli and Cutler 1996; Kadrmas et al. 2004; Dougherty et al. 2005). Transient expression of Rsu1 in Cos1 cells inhibited growth factor-induced Jun kinase activity, and the ectopic expression of Rsu1 reduced JNK phosphorylation and apoptosis in PINCH1-deleted mouse primitive endoderm cells (Montanez et al. 2012). Furthermore, Rsu1 is required for the viability of Drosophila embryos with disrupted PINCH-ILK binding (Elias et al. 2012). Hence, Rsu1-dependent regulation of stress induced kinase activity may be critical for cell survival during conditions of perturbed adhesion.
Rsu1 and the IPP proteins are widely expressed and well conserved multi-domain proteins (Li et al. 1997; Hobert et al. 1999; Zervas et al. 2001; Mackinnon et al. 2002; Clark et al. 2003; Lin et al. 2003; Kadrmas et al. 2004). Since Rsu1 associates with the IPP complex and has been shown to be required for adhesion and migration, the present work investigates the mechanistic role of Rsu1 and PINCH1 association in IPP mediated migration and signaling. In this study, we examined the effects of Rsu1 and PINCH1 depletion in adhesion, migration, FA formation and actin cytoskeleton in a non-tumorigenic mammary epithelial cell line (MCF10A). Our data demonstrate a critical role for Rsu1 and the IPP complex in proper organization of FA sites and their link to actin cytoskeleton, a requirement for cell adhesion and migration. Additionally, we revealed a unique function for Rsu1 in p38 Map Kinase signaling that appears to be independent of its interaction with the IPP complex.
Materials and methods
Cell lines
The human immortalized mammary epithelial cell line (MCF10A), 293 T and Cos1 cells used in this study were obtained from the American Type Culture Collection (Manassas, VA). MCF10A cells were maintained as described previously (Morrison et al. 2010). The 293 T and Cos1 cell lines were cultured in DMEM with low glucose supplemented with penicillin, streptomycin, glutamine and 10 % fetal bovine serum.
siRNA
Rsu1 or PINCH1 depletions were accomplished using a siRNA-mediated reverse transfection protocol as previously described (Dougherty et al. 2008). The sequences of the siRNAs (Thermo Fisher Scientific, Lafayette, CO) targeting Rsu1 and PINCH1 are: Rsu1:5′GGGAUAACGACCUGAUCUCUU-3′, Rsu1 (UTR): 5′ GAACAAAGCUCU UAUUCAAUU-3′ and human PINCH1: 5′-UGGUCUCUGCUCUUAAUAAdTdT-3′. The control siRNA is Allstars negative control siRNA (Qiagen, Valencia, CA). The siRNAs were used at a concentration of 75 nM.
Western blotting
Cell lysates were collected in RIPA or high salt buffer and processed as described previously (Dougherty et al. 2005; Galbaugh et al. 2006). The antibodies used in this study include mouse anti-talin, mouse anti-vinculin, mouse anti-β-actin, rabbit anti-actopaxin/parvin (Sigma-Aldrich, St. Louis, MO), mouse anti-paxillin, mouse anti-PINCH1, mouse anti-caveolin, mouse anti-FAK, mouse anti-β1 integrin, mouse anti-α5 integrin, mouse anti-αV, mouse anti-Rac1 (BD Biosciences, San Diego, CA), anti-phospho FAK Y397, rabbit anti-phospho-VASP Ser 157, rabbit anti-phospho-cofilin Ser3, rabbit anti-phospho-p38 Thr180/Tyr182, rabbit anti-p38, rabbit phospho-ATF2, rabbit phospho-cJun, (Cell Signaling Technologies, Danvers, MA), rabbit anti-ILK (Millipore, Billerica, MA), mouse anti-α6 integrin, mouse anti-α tubulin, mouse anti-phospho-ERK (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-PINCH1 (GenWay Biotech, San Diego, CA). The coronin 1B antibody (Cai et al. 2008) and the anti-amino-terminal Rsu1 antibody have been described (Dougherty et al. 2008).
Migration assay
Cells transfected with siRNA were seeded in Oris™ migration plates (Platypus Technologies, Madison, WI) at a concentration of 3×104 cells per ml. The wells of the 96-well plates contain removable stoppers that cover a central area into which cells migrate upon stopper removal. Seventy-two hours post transfection the stoppers were removed from each well and the media was replaced with 100 μl of fresh MCF10A culture media supplemented with 10 ng/ml of EGF. The migration plates were incubated in a humidified chamber (37 °C, 5 % CO2) for 24 h to allow cell migration. Quantitation was performed by staining cells with crystal violet and reading absorbance at 570 nM. Alternatively Calcein AM solution (10 ng/ml) was added to the cells, incubation continued for 30 min and the fluorescence signal in the central detection zones was measured by blocking all but the central part of the well with a mask (OrisR) on a plate reader (485/520 nm excitation/emission filters). The migration of cells was calculated as the mean of 4 wells minus an internal control (stoppers removed just prior to staining). Cells were photographed at the initiation and conclusion of the migration period.
The viability of MCF10A cells was measured by analyzing changes in the reduction of alamar blue (AbD Serotec, Raleigh, NC). Ninety-six hours post-transfection, alamar blue was added to each well of transfected cells (10 % of total volume) and plates were incubated at 37 °C, 5 % CO2 for 6 h. Viability was measured by calculating the percentage reduction of alamar blue using absorbance at a wavelength of 570 nm and 630 nm according to manufacturer’s protocol.
Adhesion assay
Adhesion was measured as previously described (Dougherty et al. 2005). In brief, MCF10A cells were transfected with a control, Rsu1 or PINCH1 siRNA. At 96 h post-transfection, cells were harvested and replated in a 96 well plate at a concentration of 5×104 cells per ml. At 10 min intervals unattached cells were removed and the wells washed once with PBS prior to fixation with 3.7 % formaldehyde. The cells were stained with crystal violet followed by reading absorbance at 570 nM.
Measure of cell spreading
siRNA transfected cells were seeded on fibronectin coated coverslips and fixed with paraformaldehyde 96 h post transfection. Images of cells were taken with a Zeiss 710 Confocal Laser Scanning Microscope. Cell spreading was determined by measuring the length of the cells horizontally and vertically in μm units using the LSM Image Browser. The results are displayed as the mean of 30 cells + SE. Significance was measured by Student’s t-test.
Statistical analysis
The data is displayed as mean +/− SE. Significance was measured by Student’s t-test.
Detection of GTP-Rac and GTP-Rho
Rac and Rho GTPase activity assays were performed as previously described (Dougherty et al. 2008). In brief, MCF10A cells were transfected with a control, Rsu1 or PINCH1 siRNA and plated on fibronectin-coated tissue culture dishes. At 96 h post-transfection cells were stimulated with EGF (50 ng/ml) for 15 min. The level of GTP-bound Rac1 was determined by a Rac1 effector bead binding assay (Cytoskeleton Inc., Denver Colorado). The cell lysates were collected and bound to the glutathione beads loaded with the GST-fusion of the Rac1/cdc42 binding domain of Pak. The bound Rac1, “active Rac1”, was detected by western blot with anti-Rac1 monoclonal antibody and compared to the amount of GDP- + GTP-bound Rac1, “total Rac1”, in aliquots of lysates removed prior to bead binding. The amount of active Rac1 was quantified by densitometry.
Immunofluorescence microscopy
MCF10A cells were seeded on fibronectin coverslips (BD Transduction, BD Biosciences, San Diego, CA), and assayed for immunofluorescence as previously described (Dougherty et al. 2008). In brief, cells were rinsed in phosphate buffered saline (PBS), and then fixed with cold methanol for 30 min at −20 °C or with 4 % paraformaldehyde for 15 min at room temperature (RT). Methanol-fixed cells were washed three times in PBS and then blocked in 4 % BSA for 1 h at RT or 4 °C overnight. Paraformaldehyde fixed cells were washed three times in PBS and incubated in 0.5 % Triton X-100 for 10 min. After permeabilization, cells were washed three times in PBS and blocked in 4 % BSA. Primary and secondary antibodies were diluted in 4 % BSA. Anti-amino-terminal Rsu1 rabbit polyclonal, mouse anti-talin, mouse anti-vinculin, TRITC-phalloidin (Sigma-Aldrich, St. 20 Louis, MO), mouse anti-paxillin, mouse anti-caveolin (BD Transduction, BD Biosciences, San Diego, CA), rabbit anti-Myc Tag (Cell Signaling Technologies, Danvers, MA), and mouse anti-β1 integrin antibodies were used for immunofluorescence analysis. Alexa-Fluor conjugated antibodies were used as secondary antibodies (Molecular Probes, Invitrogen, Eugene, OR). Coverslips were mounted on slides with ProLong Gold antifade reagent (Molecular Probes, Invitrogen, Eugene, OR). A Zeiss 710 Confocal Laser Scanning Microscope was used for these studies. Images were captured as Z-stacks comprised of the number of slices recommended by the LSM software. All channels were collected with the same optical unit setting. Data analysis was performed using the Zeiss LSM Image Browser. For each slide, images were obtained from random fields. Representative images were selected that displayed the phenotype consistent with the majority (over 90 %) of the random fields. Fluorescence intensity was obtained using the Zeiss 2009 software. The mean fluorescence intensity was calculated by measuring the fluorescence of vinculin in an area of 236 μm2 minus the background. Data is displayed in arbitrary units.
Co-immunoprecipitation
Immunoprecipitations of 293 T cells were performed as previously described (Dougherty et al. 2005). The immunoprecipitates were collected with a mouse anti-Myc Tag (9E10) (Roche, Indianapolis, IN) and a rat anti-HA antibody (Roche, Indianapolis, IN).
Immunoprecipitates of Rsu1-myc in MCF10A cells were collected with rabbit anti-Myc Tag antibody cross-linked to agarose beads (Sigma-Aldrich, St. Louis, MO). PINCH1 was detected with mouse anti-PINCH1 (Millipore, Billerica, MA).
MCF10A cell line construction
An Rsu1 mutant that does not bind to PINCH1 (N92D) was created by site directed mutagenesis. Co-immunoprecipitation and yeast two hybrid assays were performed as previously described to confirm that the mutant does not bind to PINCH1 as described previously (Dougherty et al. 2005). For retroviral expression cDNA encoding WT Rsu1-myc and N92D-Rsu1-myc were introduced into pBABE puromycin vectors. The vectors were transfected into packaging cell lines and virus was harvested 72 h post-transfection and filtered through a 0.45 μM filter (Millipore, Billerica, MA). MCF10A cells were infected with the virus expressing either the wt-Rsu1-myc, N92D-Rsu1-myc or empty vector. Cells were grown in 1 μg/ml of puromycin for 7 days to select for vector containing cells. Individual clones were picked and populations of puromycin selected cells were screened by western blot for expression of vector encoded protein.
The MCF10A transduced cell lines were transfected with a control or Rsu1 (UTR) siRNA and seeded into a migration plate and fibronectin coverslips for migration and immunofluorescence studies as described above. The coverslips were processed for immunofluorescence and lysates were collected for western blot analysis.
Results
Rsu1 and PINCH1 are required for MCF10A cell adhesion, cell spreading and migration
Rsu1 and PINCH1 function in cell adhesion and spreading (Dougherty et al. 2005; Xu et al. 2005; Ito et al. 2010), and they are required for cell migration (Simpson et al. 2008). A comparison of the individual contributions of Rsu1 and PINCH1 proteins to the processes of adhesion, migration, growth and survival of MCF10A cells was performed using siRNA-mediated depletion. Western blot analysis of cell lysates revealed that siRNA-mediated targeting of Rsu1 and PINCH1 for 72 h in MCF10A cells decreased their respective protein levels by 80 %. In agreement with previous studies, the depletion of Rsu1 also resulted in a substantial reduction in PINCH1 levels whereas the decrease in Rsu1 resulting from PINCH1 depletion was not as significant (Dougherty et al. 2005) (Fig. 1). MCF10A cells transfected with Rsu1 or PINCH1 siRNA were compared to control siRNA transfected cells for cell attachment to substrate. Consistent with previous studies, the reduction of Rsu1 and PINCH1 proteins decreased adhesion of MCF10A cells to tissue culture plastic with the loss of PINCH1 resulting in a more severe phenotype (Fig. 1a). However, when the effect of Rsu1 or PINCH1 level on cell spreading was examined the depletion of Rsu1 had the same impact as PINCH1 depletion (Fig. 1b). To quantify cell spreading, the length and width of individual cells were measured and used to calculate means for each population. This analysis revealed similar reductions in spreading by both length and width measurements following depletion of either Rsu1 or PINCH1.
Rsu1 and PINCH1 are required for MCF10A cell adhesion and migration. MCF10A cells were transfected with Rsu1, PINCH1 or negative control siRNA. a. Adhesion. At 96 h post transfection cells were trypsinized and tested for adhesion to tissue culture plastic. At times post plating, adherent cells were fixed and stained with crystal violet and adhesion was determined by reading absorbance at 570 nM. All time points were done in triplicates. The reduction in cell adhesion at 10, 20 and 30 min for Rsu1 and PINCH1-depleted cells was compared to Control cells, the result was significant at p ≤ 0.01. b. Spreading. MCF10A transfected cells were plated on fibronectin-coated coverslips. The cells were fixed and stained for immunofluorescence using phalloidin and anti-vinculin antibodies at 96 h post transfection. Cell spreading was determined as a measure of the length and width of cells at longest and widest point in μm. The results are displayed as the mean of 30 cells + SE. The length and width of Rsu1 or PINCH1-depleted cells were significantly reduced compared to the Control cells, p ≤ 0.001 for each comparison. c. Migration. siRNA transfected cells were seeded in OrisR migration plates and incubated 72 h. The inserts were removed, fresh media was added and migration plates were incubated at 37 °C for an additional 24 h. Cell migration was documented by microscopy/photography and quantification was performed by staining cells with calcein and reading absorbance with a template for the field of migration at 570 nm. The results are displayed as the mean of 4 independent wells + SE. The value of an internal control (IC) was subtracted from the values of all individual wells. Rsu1 and PINCH1 depleted cells differ from control with * p ≤ 0.05 and ** p ≤ 0.01 respectively. The cell viability was determined using Alamar blue. Viability was normalized to a 100 % for the Control siRNA transfected cells, and Rsu1- and PINCH1-depleted cells displayed 99 % and 97 % of control viability respectively. d. Western Blot. Verification of Rsu1 and PINCH1 reduced expression was confirmed by Western blot analysis. For quantification densitometry was performed using the Multi Gauge V3.0 software with actin as a loading control. Rsu1 and PINCH1 expression was normalized to actin. All statistical analysis for parts a- c was performed using Student T-test
MCF10A cell migration is an EGF-dependent process (Simpson et al. 2008). To analyze the effect of Rsu1 and PINCH1 expression on cell migration a two-dimensional migration assay was employed. The assay allowed siRNA transfected MCF10A cells to adhere to substrate, grow to confluency and then migrate on tissue culture plastic in wells where removable stoppers covered a round central area (migration zone). The control, Rsu1 or PINCH1 siRNA transfected cells were plated in wells and incubated for 72 h at which time the reduction in target proteins was evident and the cells had reached confluency. The stoppers were removed and EGF-dependent cell movement into the central zone was initiated. After 24 h the migration of cells was quantified using calcein staining and detection of fluorescence in the zone of migration. Cells depleted of PINCH1 exhibited a 90 % reduction in EGF-dependent cell migration while Rsu1 depletion resulted in inhibition but to a lesser degree. The microscopic images of the cells at the end of the migration period are shown and illustrate the differences among the siRNA transfected cells compared to control wells with no migration (Fig. 1c). There was no difference in the viability of Rsu1 or PINCH1 depleted cells compared to control siRNA transfected cells based on Alamar blue assay.
Disorganized focal adhesion staining in Rsu1 and PINCH1 depleted cells correlates with loss of stress fibers and elevated expression of actin binding proteins
Both Rsu1 and PINCH1 localize to focal adhesions. In an effort to elucidate the mechanism by which Rsu1 and PINCH1 depletion decreased cell adhesion and migration, we examined the impact of Rsu1 and PINCH1 expression on focal adhesions and focal adhesion protein expression in MCF10A cells. The changes in the distribution and localization of focal adhesion proteins were revealed by confocal microscopy of MCF10A cells transfected with Rsu1 or PINCH1 siRNA compared to those transfected with a non-targeting control siRNA. Depletion of Rsu1 or PINCH1 in MCF10A cells altered the distribution and localization of the FA proteins vinculin, paxillin and talin (Fig. 2a). Control siRNA treated cells showed a peripheral and highly organized staining of focal adhesion proteins while the Rsu1 or PINCH1 depleted cells exhibited retraction of the cell body and disorganized focal adhesion protein staining throughout the cytoplasm. Additionally, upon reduction of Rsu1 or PINCH1 levels MCF10A cells exhibited disorganized caveolae and the loss of visible β1 integrin complexes demonstrating that Rsu1 and PINCH1 are required for proper formation of focal adhesion sites and caveolae (Fig. 2a).
The distribution of common focal adhesion proteins in MCF10A cells is dependent on Rsu1 and PINCH1. MCF10A cells were transfected with Rsu1, PINCH1 or negative control siRNA and plated on fibronectin-coated coverslips. a. At 96 h post transfection the cells were fixed and examined by immunofluorescence using anti-paxillin, anti-vinculin, anti-talin, anti-β1 integrin and anti-caveolin antibodies. Nuclei were counterstained with DAPI. Scale bar 10 μm. b. Lysates of MCF10A cells transfected with a Rsu1, PINCH1 or negative control siRNA were harvested 96 h post transfection and examined for the expression of Rsu1, PINCH1, ILK, parvin, caveolin, paxillin, vinculin, talin, integrins (α5, α6, αV, β1), p-FAK and FAK by western blotting. Actin was used as loading control
However, the loss of focal adhesion formation in Rsu1 or PINCH1 depleted MCF10A cells did not correlate with a decrease in adhesion protein expression. The levels of the focal adhesion proteins (paxillin, vinculin, talin, FAK), integrins (α6, α5, αV, β1), and caveolin were determined by western blot analysis. Rsu1 and PINCH1 knockdown did not affect the expression of the proteins with the exception of an elevation in β1 integrin, which was observed upon both Rsu1 and PINCH1 knockdown. The level of FAK increased following PINCH1 depletion, but phosphorylated FAK (Y397) levels were reduced by 90 % in PINCH1-depleted cells and by half in the absence of Rsu1 (Fig. 2b). The elevation of β1 integrin and FAK may be a compensatory response of Rsu1- and PINCH1-depleted cells to the block in adhesion or it may represent a failure of degradation and recycling of these proteins. However, the analysis of endosomal markers and dextran uptake by Rsu1- or PINCH1-deleted cells did not reveal significant changes compared to control siRNA-transfected cells (Supplementary Figure 1).
Phalloidin labeling was used to assess changes in actin polymerization in response to reduction of Rsu1 or PINCH1. Depletion of either protein resulted in the loss of stress fibers and disorganized actin cytoskeleton staining (Fig. 3a) and correlated with loss of FAs in Rsu1- and PINCH1-depleted cells as revealed by vinculin staining. We next investigated the expression of actin binding proteins to explore differences that could explain the changes seen in the actin cytoarchitecture of Rsu1- and PINCH1-depleted cells. Depletion of PINCH1, and to a lesser degree Rsu1, resulted in an increase in the phosphorylation of VASP and cofilin on sites that control their actin polymerization/capping activity (Fig. 3b). The constitutive phosphorylation and the change in the localization and distribution of actin regulatory proteins that occurred in PINCH1-depleted cells suggested that loss of PINCH1 destabilizes the IPP complex leading to the altered regulation of actin polymerization. In this and the other evaluations of adhesion and actin cytoskeleton regulation (Supplementary Figure 2) the observation that reduction of PINCH1 levels produced significant inhibition of adhesion-related changes, and that Rsu1 levels showed an intermediate change, suggest that these functions are PINCH1 and IPP dependent. Only cell spreading appears to be highly altered by loss of Rsu1 as well as PINCH1. Hence, control of spreading may be either very sensitive to the level of PINCH1 or it may be regulated by Rsu1 function.
Disorganized focal adhesion staining correlates with loss of actin stress fibers in Rsu1 and PINCH1 depleted MCF10A cells. MCF10A cells were transfected with Rsu1, PINCH1 or negative control siRNA and plated on fibronectin-coated coverslips. a. At 96 h post transfection the cells were fixed and examined by immunofluorescence using TRITC-phalloidin and an anti-vinculin antibody. Nuclei were counterstained with DAPI. Scale bar 10 μm. b. Lysates of MCF10A cells transfected with a negative control, Rsu1 or PINCH1 siRNA were harvested 96 h post transfection and examined for the expression of Rsu1, PINCH1, phospho-VASP and phospho-cofilin by western blotting. Tubulin was used as loading control
The binding of Rsu1 to PINCH1 is required for complete restoration of adhesion and migration in Rsu1 knockdown cells
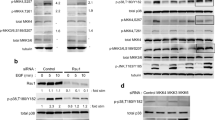
Rsu1 is linked to the IPP complex via binding to PINCH1 and reductions in Rsu1 resulted in concomitant decrease in PINCH1 protein. The studies presented above suggest that the corresponding decreases in PINCH1 levels observed in Rsu1-depleted cells may be responsible for changes in adhesion and migration, but that signaling events affecting cell spreading may also be controlled by Rsu1 independently of PINCH1 binding. Hence, the ability of Rsu1 to contribute to the regulation of adhesion, migration, and spreading independent of its binding to the PINCH1 and association with the IPP complex was addressed. A Rsu1 mutant that does not bind to PINCH1 (Rsu1-N92D) was identified and employed to determine the necessity of PINCH1 binding for rescue of adhesion and migration in Rsu1-depleted cells. Moreover, this mutant was used to detect a Rsu1 function(s) independent of the IPP complex. Several single amino acid mutations in Rsu1 were initially tested for PINCH1 binding in a GAL4 yeast two hybrid analysis. The single amino acid mutation N92D was sufficient to prevent the binding of Rsu1 to PINCH1 (Supplementary Table 1). Additionally, the ability of the Rsu1-N92D mutant to associate with PINCH1 in mammalian cells was examined by transient expression and co-immunoprecipitation studies in 293 T cells, which showed that immunoprecipitation of HA-tagged PINCH1 also co-precipitated myc epitope-tagged wt-Rsu1-myc but not N92D-Rsu1 (Fig. 4c). Since the adhesion and migration studies were conducted in MCF10A cells, we transduced MCF10A cell lines to express myc-tagged wt-Rsu1 or the N92D-Rsu1 using a pBABE-puromycin retroviral vector. The infectant MCF10A clones were selected in puromycin and analyzed for expression of the vector-encoded protein. As shown in Fig. 4d, the wt-Rsu1-myc but not N92D-Rsu1-myc protein bound and co-precipitated endogenous PINCH1 from MCF10A infectant cell lysates.
Rsu1 - PINCH1 interaction is required to promote the formation of mature focal adhesions in MCF10A cells. MCF10A cell lines infected with pBABE control vector or expressing wt-Rsu1-myc or N92D-Rsu1-myc were depleted of endogenous Rsu1 by transfection with endogenous Rsu1 specific siRNA. The cells were plated on fibronectin coverslips and compared to those transfected with negative control siRNA. a. Cells were fixed at 96 h post transfection and assayed for Rsu1 by immunofluorescence b. Cells were fixed and stained with anti-myc tag and anti-vinculin antibodies. Nuclei were counterstained with DAPI. Scale bar 10 μm. Zen 2009 software was used for fluorescence intensity measurements. Fluorescence values are expressed in arbitrary units. c. Lysates of 293 T cells cotransfected with plasmid encoding HA-PINCH1 and wt-Rsu1-myc or N92D-Rsu1-myc were immunoprecipitaed with anti-HA (rat) antibody. The immunoprecipitates and sample of lysates were analyzed by western blot with anti-myc tag (mouse) antibody for presence of myc-tagged Rsu1. The blot was subsequently reacted with antibody to Rsu1 to demonstrate that the endogenous Rsu1 co-precipitated with HA-PINCH1. d. Lysates from MCF10A cells infected with pBABE, wt-Rsu1-myc or N92D-Rsu1-myc were immunoprecipitated with anti-myc IgG (rabbit) cross linked to agarose beads. The immunoprecipitates and sample of lysates were analyzed by western blot with anti-PINCH (mouse) antibody for presence of co-precipitated endogenous PINCH1 and anti-Rsu1 (rabbit) for endogenous and myc-tagged Rsu1
To examine the requirement for Rsu1 binding to PINCH1 in cell adhesion and migration, the wt-Rsu1-myc and N92D-Rsu1-myc MCF10A cells were depleted of endogenous Rsu1 and co-stained for vinculin and myc-tagged Rsu1 (Fig. 4b). siRNA targeting the 3’UTR region of the Rsu1 mRNA was transfected into the infectant clones to reduce the endogenous Rsu1 while allowing expression of the proteins from the retroviral vectors. The elimination of endogenous Rsu1 from the pBabe vector control cells decreased the number and size of focal adhesions as expected. Expression of wt-Rsu1-myc was accompanied by restoration of FAs in Rsu1 depleted cells, while the N92D-Rsu1-myc mutant displayed fewer and more disorganized vinculin staining following the elimination of endogenous Rsu1 (Fig. 4a). However, expression of either wt-Rsu1 or N92D-Rsu1 restored the levels of PINCH1 that were decreased following the depletion of endogenous Rsu1 (Fig. 5).
Rsu1-PINCH1 interaction is required for MCF10A migration. MCF10A cells expressing Rsu1-myc or N92D-Rsu1-myc were transfected with negative control siRNA or siRNA specific for endogenous Rsu1 and compared to siRNA transfected pBABE control MCF10A cells. a. Adhesion. At 96 h post transfection the cells were trypsinized and tested for adhesion to tissue culture plastic. At times post plating, cells were fixed and stained with crystal violet and adhesion was determined by reading absorbance at 570 nM. All time points were done in triplicates. Differences between Rsu1 siRNA-transfected pBABE cells or cells expressing wt-Rsu1-myc compared to respective negative control transfected cells were significant at p ≤ 0.01 for 10, 20 and 30 min timepoints. b. Spreading. Cells plated on fibronectin coverslips were fixed at 96 h post transfection and stained with anti-myc antibody. Cell spreading was determined as a measure of the length and width of cells at longest and widest point in μm. The results are displayed as the mean of 30 cells + SE. The mean length and width of Rsu1-depleted pBABE cells was significantly reduced compared to wt-Rsu1-myc or N92day-Rsu1-myc expressing cells, p ≤ 0.001. c. Migration. siRNA treated cells were seeded in migration plates and incubated for 72 h prior to removal of inserts. Migration plates were incubated at 37 °C for an additional 24 h. Cell migration was documented by microscopy/photography and quantification was performed by fluorescently staining cells with Calcein AM and reading fluorescence with a template isolating the field of migration at 485/520 nm. The results are displayed as the mean of 4 independent wells + SE. Migration of pBABE cells transfected with Rsu1 siRNA was significantly reduced compared to the wt-Rsu1-myc expressing cells transfected with Rsu1 siRNA at p ≤ 0.01. N92D-Rsu1-myc expressing cells exhibited reduced migration compared to pBABE control cells at p ≤ 0.01. d. Western Blot. Lysates of cells were harvested at 96 h post transfection and examined for the expression of Rsu1, PINCH1 and ILK by Western blotting. Actin was used as loading control
Following depletion of endogenous Rsu1, MCF10A cells expressing wt-Rsu1-myc and N92D-Rsu1-myc were tested for adhesion. Only the wt-Rsu1-myc completely restored the cell adhesion decreased by the loss of endogenous Rsu1, suggesting that Rsu1-PINCH1 binding was necessary for efficient adhesion (Fig. 5a). Next the MCF10A infectant cells were depleted of endogenous Rsu1 and tested for ability of the vector encoded proteins to restore migration in OrisR migration plates as described above. Again, expression of the wt-Rsu1-myc in the absence of endogenous Rsu1 resulted in restoration of migration while the N92D-Rsu1-myc mutant did not restore migration in Rsu1-depleted cells. In addition, the N92D-Rsu1-myc inhibited migration even in the presence of endogenous Rsu1, suggesting a dominant-negative function in MCF10A cells (Fig. 5b). However, both the wt-Rsu1-myc and the N92D-Rsu1-myc restored cell spreading (Fig. 5c). Together these results indicate that the binding of Rsu1 to PINCH1 is required for proper formation of functional focal adhesion sites as well as the processes of adhesion and migration but not for cell spreading. While expression of N92D-Rsu1-myc restored levels of PINCH1 (Fig. 5d) this was not sufficient to compensate for the absence of Rsu1-PINCH1 binding. Because spreading is an EGF-dependent process in MCF10A cells, the role of Rsu1 is likely involved in linking IPP complex to EGF-R signaling.
The EGF-dependent activation of p38 kinase requires Rsu1, but not Rsu1-PINCH1 association
Because the expression, localization and phosphorylation of Rsu1 and members of the IPP complex have an impact on Rac1 and RhoA activation and their signaling pathways, we examined the effect of Rsu1 or PINCH1 depletion on Rac1-GTP and RhoA-GTP levels in MCF10A cells by pulldown activity assays (Vasaturo et al. 2000; Dougherty et al. 2008). As expected, the constitutive levels of Rac-GTP were reduced in Rsu1- and PINCH1-depleted cells compared to control cells. However, the Rsu1- or PINCH1-depleted cells retained the ability to activate Rac1 GTP loading in response to EGF stimulation (Fig. 6a). In other experiments Rho was activated in stimulated cells as well (data not shown).
The inhibitory effect of Rsu1 and PINCH1 depletion on Rac1 activation was mitigated by EGF but p38 Map Kinase activation requires Rsu1. a. Rac activation. MCF10A cells were transfected with Rsu1, PINCH1 or negative control siRNA and plated on fibronectin-coated plates. The cells were grown for 72 h in complete media then starved of EGF for 24 h. Untreated cells or cells exposed to EGF (50 ng/ml) for 10 min were lysed and the amount of Rac-GTP was determined by pulldown assay using GST-Pak-RBD beads. The amount of bead-bound Rac (Rac-GTP) as well as the amount of total Rac in a sample of lysate was determined by western blot with anti-Rac antibody. The blots were quantified to determine the level of active Rac. b. Lysates were prepared from MCF10A cells transfected with Rsu1, PINCH1 or control siRNA and maintained for 96 h in growth media. Equal amounts of the lysates were analyzed by western blotting for the level of Rsu1, PINCH1, p38 Map Kinase and phosphorylated p38 (Thre180, Tyr182). c. Lysates were prepared from MCF10A cells transfected with Rsu1, PINCH1 or control siRNA and maintained for 72 h in growth media, starved of EGF for 24 h, stimulated with EGF (100 ng/ml) for times indicated. Equal amounts of the lysates were analyzed by western blotting for the level of phosphorylation of p38, ATF2, c-Jun proteins. d. Lysates were prepared from MCF10A pBABE vector control, wt-Rsu1-myc, and N92D-Rsu1-myc expressing cell lines that had been transfected with Rsu1 or negative control siRNA and maintained for 96 h in growth media. The lysates were analyzed by western blot for p38 phosphorylation (Thr180, Tyr182)
Another measure of MCF10A cell functionality is the ability to form mammary acini when cultured in Matrigel. While Rac1 is not required for this process, the deletion of β1 integrin has a profound effect, and the deletion of ILK impairs acini formation (Akhtar and Streuli 2013). However, in the absence of PINCH1 or Rsu1 the MCF10A cells were able to form acini and clear the luminal region as indicated by caspase staining (Supplementary Figure 3). We also examined the requirement for Rsu1-PINCH1 binding in the formation of acini. MCF10A cells expressing wt-Rsu1-myc or N92D-Rsu1-myc were transfected with a control or Rsu1 siRNA and plated on matrigel coated slides. The formation of acinar structures was evaluated by immunofluorescent staining with antibodies for Rsu1, caspases and IPP proteins. Again, the ability of the cells to form acini was not significantly affected by endogenous Rsu1 depletion or the expression of N92D-Rsu1-myc. Despite the dramatic phenotypic changes observed in Rsu1 depleted cells, these data demonstrated that neither PINCH1 nor Rsu1, nor the interaction of Rsu1-PINCH1, are absolutely required for acini formation by MCF10A cells.
The loss of cell adhesion in MCF10A cells activates signal transduction pathways leading to phosphorylation of the MAP kinases p38 kinase and Jun kinase (Wen et al. 2011), as does the stimulation of receptor tyrosine kinases, including EGF-R. The cells depleted of Rsu1 and PINCH1 were examined both at homeostasis (Fig. 6b) and following stimulation with EGF (Fig. 6c) to determine if common signal transduction pathways were altered by reduction of Rsu1 or PINCH1. Basal levels of phosphorylation of many kinase pathways were similar in control and Rsu1 or PINCH1 depleted cells. However, the decreased level of Rsu1, but not PINCH1, corresponded to a decrease in phosphorylation of p38 Map Kinase as revealed using antibody that recognized p38 dually phosphorylated on Thr180 and tyr182 (Fig. 6b). Our analysis of the p38 isoforms in MCF10A cells agreed with other studies and revealed that the cells expressed p38α, β, γ and δ (Wen et al. 2011). In response to EGF stimulation, the Rsu1-depleted cells exhibited reduced phosphorylation of p38 Map kinase but PINCH1 depletion did not block its stimulation in adherent cells (Fig. 6c). Further experiments revealed that the failure to activate p38 was primarily restricted to growth factor-dependent activation pathways as physical detachment of the Rsu1-depleted MCF10A cells exhibited activated p38 as in control cells (data not shown). Moreover, the decreased activation of p38 in response to EGF stimulation also resulted in failure to activate phosphorylation of ATF2, a known target of p38 kinase activity. The inhibition of ATF2 activation occurred under conditions where c-Jun was phosphorylated (Fig. 6c). Hence, the data suggest that EGF-induced activation of p38 kinase required Rsu1 but not PINCH1.
This finding implied that Rsu1 like Nck2, another PINCH1 binding protein, might be essential for the responses to growth factors that require integrin engagement. To discriminate between the function of Rsu1 alone and Rsu1 bound to PINCH1 in the p38 signaling pathway, MCF10A cells expressing myc-tagged wt-or N92D-Rsu1 were depleted of endogenous Rsu1. While p38 kinase was phosphorylated in response to EGF in the MCF10A cells expressing wt-Rsu1-myc, the expression of the N92D-Rsu1-myc protein resulted in constitutive p38 phosphorylation even in the absence of EGF stimulation. This suggested that Rsu1 was essential for activation of p38 kinase in MCF10A cells and that its association with PINCH1 and the IPP complex was not required. Based on the ability of N92D-Rsu1 to activate p38 phosphorylation, the association with PINCH1 and the IPP complex may even inhibit the function of Rsu1 in p38 activation.
Discussion
The present work investigated the requirement of the Rsu1-PINCH1 interaction in the regulation of cell adhesion, spreading and migration in MCF10A mammary epithelial cells. Evidence from previous studies established that Rsu1-PINCH1 binding was required for cell adhesion and PINCH1 functionality (Zhang et al. 2002a, b; Dougherty et al. 2005; Legate et al. 2006; Dougherty et al. 2008; Stanchi et al. 2009; Zervas et al. 2011). We have now shown that independent depletion of Rsu1 and PINCH1 resulted in decreased cell adhesion, spreading and migration in MCF10A cells. This change appears to be a direct consequence of a disruption in the functionality of focal adhesion sites as demonstrated by the altered distribution and localization of the FA proteins paxillin, vinculin, talin, and β1 integrin. In addition, the levels of p-FAK (Tyr 397) were significantly reduced in Rsu1 and PINCH1 depleted cells. Because FAK plays critical roles in cell spreading, survival, proliferation and migration (Petit and Thiery 2000; Serrels et al. 2007; Dumbauld et al. 2010) and is essential in FA signaling and turnover (Ilic et al. 1995; Cary et al. 1996), we conclude that the absence of its phosphorylation reduced the assembly of FA proteins at adhesion sites.
Most phenotypes associated with decreased levels of either Rsu1 or PINCH1 were more severe in the PINCH1-depleted cells. Moreover, depletion of Rsu1 caused significant reduction in PINCH1, while PINCH1 depletion resulted in only a modest reduction in Rsu1. This observation is in agreement with other studies and implies that one Rsu1 function in the IPP complex is the regulation of PINCH1 levels and PINCH1 stabilization (Kadrmas et al. 2004; Dougherty et al. 2005; Elias et al. 2012). Hence, the phenotype observed in Rsu1-depleted cells may be partially due to decreased PINCH1 levels.
In adherent cells, integrins prevent lipid raft internalization by sequestering caveolin at the FA sites and, conversely, cells undergoing detachment release caveolin from the FAs (Caswell et al. 2009). ILK function is required for normal caveolar trafficking (Meyer et al. 2005; Wickstrom et al. 2010a). Hence, the localization and distribution of β1 integrin in “endosome like structures” and the disorganized caveolae observed upon Rsu1 and PINCH1 depletion are likely linked to changes in PINCH1-ILK function. While ILK expression was slightly affected by Rsu1 reduction, PINCH1 depletion significantly decreased ILK levels which could translate into a reduction in the number and functionality of ILK molecules. Similarly, Rsu1 and PINCH1 depleted cells displayed a loss of actin stress fibers. PINCH1 depletion also caused increased phosphorylation of actin regulatory proteins VASP and cofilin, which suggests a mechanism for the defect in actin cytoskeleton remodeling. The loss of actin stress fibers are likely the outcome of defective ILK-parvin association resulting from fewer ILK molecules available for parvin binding. Although ILK associates with PINCH1, the ILK-parvin binding is critical for actin mediated functions in adhesion (Fukuda et al. 2009; Wickstrom et al. 2010b). Since parvins function in the regulation of actin cytoskeleton dynamics and FA turnover, disruption of ILK -PINCH1 interaction contributes to a more severe defect in adhesion and migration in PINCH1-depleted compared to Rsu1-depleted cells.
While most of the phenotypes exhibited by Rsu1-depleted cells were not as severe as those lacking PINCH1, cell spreading was equally affected by Rsu1 or PINCH1 depletion. To address the mechanism behind this finding, we asked if Rsu1 has a function independent of IPP signaling by the generating an Rsu1 mutant (N92D) that fails to bind PINCH1. Reconstitution of Rsu1-depleted cells with the N92D mutant of Rsu1 did not restore FAs and migration, indicating that binding of Rsu1 to PINCH1-ILK-parvin is required for the proper regulation of adhesion and migration. However, the mutant protein did restore spreading in MCF10A cells otherwise defective due to the depletion of the endogenous Rsu1. This also suggests that the smaller focal contacts observed in the N92D-Rsu1 expressing cells promoted sufficient focal adhesion protein interaction for actin-based spreading. However, the dissolution and reformation of adhesion sites needed for migration was impaired and the Rsu1-PINCH1 structures appear to be required for simple adhesion as well. In support of this analysis, Rsu1-PINCH1 interaction is required for formation of focal adhesions strong enough to rescue muscle cell attachment defects in Drosophila (Pronovost et al. 2013).
Previous work demonstrated that Rsu1 levels altered Jun kinase and p38 kinase activity (Masuelli and Cutler 1996; Kadrmas et al. 2004; Dougherty et al. 2005). p38 MAPK signaling is required for cell spreading and migration in response to diverse signals (Frey et al. 2004; Pichon et al. 2004; Dobreva et al. 2006; Varon et al. 2008). Hence, the observation that depletion of Rsu1, but not PINCH1, blocked growth factor induced p38 phosphorylation in MCF10A cells suggested a unique function for Rsu1. The wt-Rsu1, and also the N92D-Rsu1 mutant, restored p38 activity in Rsu1 depleted cells supporting the finding of an Rsu1 function independent of IPP signaling. Furthermore, the elevated phospho-p38 and the recovery of cell spreading resulting from expression of the N92D-Rsu1 mutant suggested that Rsu1 contributes to cell spreading by promoting signaling through the p38 MAP kinase. Data from several studies supports a role for p38 in actin cytoskeletal regulation and spreading. For example, WAVE3 promotes cell motility via the p38 Map kinase pathway and MPP expression (Sossey-Alaoui et al. 2005) and signaling through p38 is required for actin polymerization and cell migration in smooth muscle cells (Pichon et al. 2004) and intestinal epithelial cells (Frey et al. 2004), suggesting that actin regulation was affected p38 activity in our system. ILK contributes to Src activation at focal adhesion sites as well as the formation of dorsal ruffles in fibroblasts (Azimifar et al. 2012) and src is an activator of p38 (Ouwens et al. 2002; Frey et al. 2004). However, our data indicate that src is phosphorylated at tyrosine 416 in Rsu1-depleted cells (data not shown) suggesting that this is not the mechanism at work in Rsu1-depleted MCF10A cells.
The elevated levels of p38 activation displayed by the Rsu1 mutant and the lack of p38 activity exhibited by the Rsu1-depleted cells supports a function for Rsu1 in promoting cell survival through a p38 mediated mechanism. p38 Map kinase is an important regulator of cell death and survival under conditions of stress, including detachment-induced stress that occurs during acini formation in MCF10A cells. In this process activated p38 is thought to be essential for apoptotic signals required for lumen clearance (Wen et al. 2011). In cells depleted of Rsu1 the defect in p38 activation was restricted to an EGF-induced activation pathway; therefore, the detachment of Rsu1-depleted cells resulted in p38 phosphorylation and allowed formation of acini exhibiting luminal clearance. However, phospho-p38 can function to activate checkpoint controls that allow cell survival under conditions of stress in MCF10A cells (Bulavin et al. 2001). While p38 is frequently associated with apoptosis, p38 and ATF2 were required for survival in embryonic liver (Breitwieser et al. 2007) and B-CLL (Ringshausen et al. 2004). This suggests that Rsu1 may function to facilitate p38 activation in response to growth factor dependent changes.
Several lines of evidence support a survival function for Rsu1. In Drosophila embryos with disruption of PINCH-ILK binding the depletion of Rsu1 resulted in lethality independently of PINCH1 localization or stabilization (Elias et al. 2012). Montanez et al. established that depletion of PINCH1 resulted in a decrease of Rsu1 in mouse primitive endoderm cells concomitant with increased JNK and Bax activation and reduced Bcl-2 levels, and introduction of Rsu1 decreased JNK activity (Montanez et al. 2012). It is not clear if Rsu1 functions to regulate p38 in these systems but it could contribute to survival by activating a p38 checkpoint control.
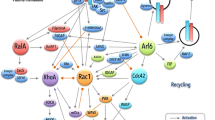
The mechanism by which Rsu1 contributes to p38 phosphorylation, or by which N92D-Rsu1 elevates it, is not known (Fig. 7). There are several relevant control points including its activation by MKK4 or MKK3 and MKK6, p38 dephosphorylation by multiple phosphatases, and subcellular localization regulated by scaffolding proteins. The identification of PP1α as a PINCH1 associated protein, which binds to a site on PINCH1 LIM5 domain close to that bound by Rsu1 (Eke et al. 2010), suggests that PP1α activity toward p38 or its upstream activators may be compromised in the absence of Rsu1-PINCH1 binding. Our current data have not adequately distinguished among the control points for activation of p38 in MCF10A cells to know which is most relevant to the role of Rsu1. Our previous studies determined that ectopic expression of Rsu1 decreased activation of Jun kinase by growth factor. Modulation of p38 at the level of a common Jun kinase and p38 activator could account for the divergent results obtained following Rsu1 depletion or ectopic expression. Hence, it is important to note that MLK3, an upstream activator of both p38 and Jun kinase, was identified as a required protein for MCF10A migration in screening analysis of thousands of adhesion proteins (Simpson et al. 2008). This finding supports the idea that the pathway leading to p38 activation is important for EGF-induced migration in MCF10A cells. Our current efforts are directed at understanding how Rsu1 regulates p38 and Jun kinase activity.
Rsu1 is a critical component for regulation of p38 Map kinase signaling from the IPP complex. Rsu1 is required for the constitutive and EGF-induced p38 signaling. The elimination of Rsu1 reduces both constitutive and EGF-induced p38 phosphorylation and the expression of N92D-Rsu1 activates constitutive p38 signaling. p38 Map kinase can be activated by detachment and this may occur via the αPix-induced Rac pathway. Dashed and dotted lines represent incompletely characterized pathways
This work highlights the role of Rsu1 and PINCH1 in adhesion and migration. Collectively, these results demonstrate a critical role for Rsu1 and IPP complex interaction in proper formation of FA sites and actin cytoskeleton remodeling. More importantly our findings revealed a unique role for Rsu1 in cell spreading and p38 activation that is independent from IPP signaling. Further work will be required to identify the mechanism by which the Rsu1 PINCH1-non-binding mutant affects p38 phosphorylation, PINCH1 stability and cell signaling.
References
Akhtar N, Streuli CH (2013) An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat Cell Biol 15:17–27
Azimifar SB, Bottcher RT, Zanivan S, Grashoff C, Kruger M, Legate KR, Mann M, Fassler R (2012) Induction of membrane circular dorsal ruffles requires co-signalling of integrin-ILK-complex and EGF receptor. J Cell Sci 125:435–448
Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D (2004) Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 432:466–472
Braun A, Bordoy R, Stanchi F, Moser M, Kostka GG, Ehler E, Brandau O, Fassler R (2003) PINCH2 is a new five LIM domain protein, homologous to PINCHand localized to focal adhesions. Exp Cell Res 284:239–250
Breitwieser W, Lyons S, Flenniken AM, Ashton G, Bruder G, Willington M, Lacaud G, Kouskoff V, Jones N (2007) Feedback regulation of p38 activity via ATF2 is essential for survival of embryonic liver cells. Genes Dev 21:2069–2082
Bulavin DV, Higashimoto Y, Popoff IJ, Gaarde WA, Basrur V, Potapova O, Appella E, Fornace AJ Jr (2001) Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 411:102–107
Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P (2010) Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer 10:858–870
Cai L, Makhov AM, Schafer DA, Bear JE (2008) Coronin 1B antagonizes cortactin and remodels Arp2/3-containing actin branches in lamellipodia. Cell 134:828–842
Cary LA, Chang JF, Guan JL (1996) Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J Cell Sci 109(Pt 7):1787–1794
Caswell PT, Vadrevu S, Norman JC (2009) Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 10:843–853
Clark KA, McGrail M, Beckerle MC (2003) Analysis of PINCH function in Drosophila demonstrates its requirement in integrin-dependent cellular processes. Development 130:2611–2621
Cutler ML, Bassin RH, Zanoni L, Talbot N (1992) Isolation of rsp-1, a novel cDNA capable of suppressing v-Ras transformation. Mol Cell Biol 12:3750–3756
Dobreva I, Waeber G, James RW, Widmann C (2006) Interleukin-8 secretion by fibroblasts induced by low density lipoproteins is p38 MAPK-dependent and leads to cell spreading and wound closure. J Biol Chem 281:199–205
Dougherty GW, Chopp T, Qi SM, Cutler ML (2005) The Ras suppressor Rsu-1 binds to the LIM 5 domain of the adaptor protein PINCH1 and participates in adhesion-related functions. Exp Cell Res 306:168–179
Dougherty GW, Jose C, Gimona M, Cutler ML (2008) The Rsu-1-PINCH1-ILK complex is regulated by Ras activation in tumor cells. Eur J Cell Biol 87:721–734
Dumbauld DW, Michael KE, Hanks SK, Garcia AJ (2010) Focal adhesion kinase-dependent regulation of adhesive forces involves vinculin recruitment to focal adhesions. Biol Cell 102:203–213
Eke I, Koch U, Hehlgans S, Sandfort V, Stanchi F, Zips D, Baumann M, Shevchenko A, Pilarsky C, Haase M, Baretton GB, Calleja V, Larijani B, Fassler R, Cordes N (2010) PINCH1 regulates Akt1 activation and enhances radioresistance by inhibiting PP1alpha. J Clin Invest 120:2516–2527
Elias MC, Pronovost SM, Cahill KJ, Beckerle MC, Kadrmas JL (2012) A crucial role for Ras suppressor-1 (RSU-1) revealed when PINCH and ILK binding is disrupted. J Cell Sci 125:3185–3194
Frey MR, Golovin A, Polk DB (2004) Epidermal growth factor-stimulated intestinal epithelial cell migration requires Src family kinase-dependent p38 MAPK signaling. J Biol Chem 279:44513–44521
Fukuda K, Gupta S, Chen K, Wu C, Qin J (2009) The pseudoactive site of ILK is essential for its binding to alpha-Parvin and localization to focal adhesions. Mol Cell 36:819–830
Galbaugh T, Cerrito MG, Jose CC, Cutler ML (2006) EGF-induced activation of Akt results in mTOR-dependent p70S6 kinase phosphorylation and inhibition of HC11 cell lactogenic differentiation. BMC Cell Biol 7:34
Hobert O, Moerman DG, Clark KA, Beckerle MC, Ruvkun G (1999) A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans. J Cell Biol 144:45–57
Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377:539–544
Ito S, Takahara Y, Hyodo T, Hasegawa H, Asano E, Hamaguchi M, Senga T (2010) The roles of two distinct regions of PINCH-1 in the regulation of cell attachment and spreading. Mol Biol Cell 21:4120–4129
Kadrmas JL, Smith MA, Clark KA, Pronovost SM, Muster N, Yates JR 3rd, Beckerle MC (2004) The integrin effector PINCH regulates JNK activity and epithelial migration in concert with Ras suppressor 1. J Cell Biol 167:1019–1024
LaLonde DP, Brown MC, Bouverat BP, Turner CE (2005) Actopaxin interacts with TESK1 to regulate cell spreading on fibronectin. J Biol Chem 280:21680–21688
Legate KR, Montanez E, Kudlacek O, Fassler R (2006) ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol 7:20–31
Li F, Liu J, Mayne R, Wu C (1997) Identification and characterization of a mouse protein kinase that is highly homologous to human integrin-linked kinase. Biochim Biophys Acta 1358:215–220
Li F, Zhang Y, Wu C (1999) Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J Cell Sci 112(Pt 24):4589–4599
Li S, Bordoy R, Stanchi F, Moser M, Braun A, Kudlacek O, Wewer UM, Yurchenco PD, Fassler R (2005) PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J Cell Sci 118:2913–2921
Liang X, Zhou Q, Li X, Sun Y, Lu M, Dalton N, Ross J Jr, Chen J (2005) PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol 25:3056–3062
Lin X, Qadota H, Moerman DG, Williams BD (2003) C. elegans PAT-6/actopaxin plays a critical role in the assembly of integrin adhesion complexes in vivo. Curr Biol 13:922–932
Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD (2002) C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol 12:787–797
Masuelli L, Cutler ML (1996) Increased expression of the Ras suppressor Rsu-1 enhances Erk-2 activation and inhibits Jun kinase activation. Mol Cell Biol 16:5466–5476
Meyer A, van Golen CM, Boyanapalli M, Kim B, Soules ME, Feldman EL (2005) Integrin-linked kinase complexes with caveolin-1 in human neuroblastoma cells. Biochemistry 44:932–938
Montanez E, Karakose E, Tischner D, Villunger A, Fassler R (2012) PINCH-1 promotes Bcl-2-dependent survival signalling and inhibits JNK-mediated apoptosis in the primitive endoderm. J Cell Sci 125:5233–5240
Morrison BL, Jose CC, Cutler ML (2010) Connective Tissue Growth Factor (CTGF/CCN2) enhances lactogenic differentiation of mammary epithelial cells via integrin-mediated cell adhesion. BMC Cell Biol 11:35
Nikolopoulos SN, Turner CE (2000) Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J Cell Biol 151:1435–1448
Ouwens DM, de Ruiter ND, van der Zon GC, Carter AP, Schouten J, van der Burgt C, Kooistra K, Bos JL, Maassen JA, van Dam H (2002) Growth factors can activate ATF2 via a two-step mechanism: phosphorylation of Thr71 through the Ras-MEK-ERK pathway and of Thr69 through RalGDS-Src-p38. EMBO J 21:3782–3793
Petit V, Thiery JP (2000) Focal adhesions: structure and dynamics. Biol Cell 92:477–494
Pichon S, Bryckaert M, Berrou E (2004) Control of actin dynamics by p38 MAP kinase - Hsp27 distribution in the lamellipodium of smooth muscle cells. J Cell Sci 117:2569–2577
Pronovost SM, Beckerle MC, Kadrmas JL (2013) Elevated expression of the integrin-associated protein PINCH suppresses the defects of drosophila melanogaster muscle hypercontraction mutants. PLoS Genet 9:e1003406
Qin J, Wu C (2012) ILK: a pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr Opin Cell Biol 24:607–613
Ringshausen I, Dechow T, Schneller F, Weick K, Oelsner M, Peschel C, Decker T (2004) Constitutive activation of the MAPkinase p38 is critical for MMP-9 production and survival of B-CLL cells on bone marrow stromal cells. Leukemia 18:1964–1970
Rosenberger G, Jantke I, Gal A, Kutsche K (2003) Interaction of alphaPIX (ARHGEF6) with beta-parvin (PARVB) suggests an involvement of alphaPIX in integrin-mediated signaling. Hum Mol Genet 12:155–167
Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fassler R (2003) Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev 17:926–940
Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, Jones GE, Frame MC (2007) Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol 9:1046–1056
Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, Brugge JS (2008) Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol 10:1027–1038
Sossey-Alaoui K, Ranalli TA, Li X, Bakin AV, Cowell JK (2005) WAVE3 promotes cell motility and invasion through the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp Cell Res 308:135–145
Stanchi F, Grashoff C, Nguemeni Yonga CF, Grall D, Fassler R, Van Obberghen-Schilling E (2009) Molecular dissection of the ILK-PINCH-parvin triad reveals a fundamental role for the ILK kinase domain in the late stages of focal-adhesion maturation. J Cell Sci 122:1800–1811
Tsuda T, Marinetti MR, Masuelli L, Cutler ML (1995) The Ras suppressor RSU-1 localizes to 10p13 and its expression in the U251 glioblastoma cell line correlates with a decrease in growth rate and tumorigenic potential. Oncogene 11:397–403
Tu Y, Li F, Wu C (1998) Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways. Mol Biol Cell 9:3367–3382
Tu Y, Li F, Goicoechea S, Wu C (1999) The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol 19:2425–2434
Tu Y, Huang Y, Zhang Y, Hua Y, Wu C (2001) A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol 153:585–598
Varon C, Rottiers P, Ezan J, Reuzeau E, Basoni C, Kramer I, Genot E (2008) TGFbeta1 regulates endothelial cell spreading and hypertrophy through a Rac-p38-mediated pathway. Biol Cell 100:537–550
Vasaturo F, Dougherty GW, Cutler ML (2000) Ectopic expression of Rsu-1 results in elevation of p21CIP and inhibits anchorage-independent growth of MCF7 breast cancer cells. Breast Cancer Res Treat 61:69–78
Velyvis A, Vaynberg J, Yang Y, Vinogradova O, Zhang Y, Wu C, Qin J (2003) Structural and functional insights into PINCH LIM4 domain-mediated integrin signaling. Nat Struct Biol 10:558–564
Wen HC, Avivar-Valderas A, Sosa MS, Girnius N, Farias EF, Davis RJ, Aguirre-Ghiso JA (2011) p38alpha signaling induces anoikis and lumen formation during mammary morphogenesis. Sci Signal 4:ra34
Wickstrom SA, Lange A, Hess MW, Polleux J, Spatz JP, Kruger M, Pfaller K, Lambacher A, Bloch W, Mann M, Huber LA, Fassler R (2010a) Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell 19:574–588
Wickstrom SA, Lange A, Montanez E, Fassler R (2010b) The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J 29:281–291
Xu Z, Fukuda T, Li Y, Zha X, Qin J, Wu C (2005) Molecular dissection of PINCH-1 reveals a mechanism of coupling and uncoupling of cell shape modulation and survival. J Biol Chem 280:27631–27637
Yamaji S, Suzuki A, Kanamori H, Mishima W, Yoshimi R, Takasaki H, Takabayashi M, Fujimaki K, Fujisawa S, Ohno S, Ishigatsubo Y (2004) Affixin interacts with alpha-actinin and mediates integrin signaling for reorganization of F-actin induced by initial cell-substrate interaction. J Cell Biol 165:539–551
Zervas CG, Gregory SL, Brown NH (2001) Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J Cell Biol 152:1007–1018
Zervas CG, Psarra E, Williams V, Solomon E, Vakaloglou KM, Brown NH (2011) A central multifunctional role of integrin-linked kinase at muscle attachment sites. J Cell Sci 124:1316–1327
Zhang Y, Chen K, Tu Y, Velyvis A, Yang Y, Qin J, Wu C (2002a) Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J Cell Sci 115:4777–4786
Zhang Y, Guo L, Chen K, Wu C (2002b) A critical role of the PINCH-integrin-linked kinase interaction in the regulation of cell shape change and migration. J Biol Chem 277:318–326
Acknowledgments
The authors would like to acknowledge the technical assistance of Laurelis Santiago and helpful discussion from members of the laboratory. The expert advice on endocytosis from Drs. Gudrun Ihrke and Michael Schell and reagents provided by them for those assays were much appreciated. The authors are grateful to Dr. Jim Bear for providing the anti-coronin antibody. The following funding agencies provided support: Congressionally Directed Medical Research Program grant W81XWH-09-2-0056 from the Wound Healing Program (to MLC), grant from the Murtha Cancer Center (to MLC) and W81XWH-10-1-0024 from the Congressionally Directed Medical Research Breast Cancer Program (predoctoral fellowship to RG-N).
The opinions expressed here are those of the authors and should not be construed as official or reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Rsu1 or PINCH1 depletion does not affected endocytic transport. MCF10A cells were transfected with Control, Rsu1 or PINCH1 siRNA and plated on fibronectin coverslips. (a) Cells were fixed at 96 hours and assayed by immunofluorescence using EEA1 and transferrin receptor antibodies. Anti-EEA1 and Anti-Transferrin receptor antibodies were a generous gift from Dr. Gudrun Ihrke. Nuclei were counterstained with DAPI. (b) Changes in endocytosis were measured by using the Dextran uptake assay. A FITC-labeled Dextran was a generous gift from Dr. Gudrun Ihrke. Transiently transfected cells were washed with pre-warmed PBS and then incubated with FITC-Dextran at 37ºC for 1 hour. This time point allows for the detection of changes in endocytosis in late endosomes. Following the one-hour incubation, cells were fixed and processed for immunofluorescence. Co-staining with Dextran was done using a mouse anti-β1 integrin antibody. Scale bar 10μm (JPEG 58 kb)
Supplementary Figure 2
Disorganized focal adhesion staining correlates with loss of actin stress fibers in Rsu1 and PINCH1 depleted cells. MCF10A cells were transfected with a Control, Rsu1 or PINCH1 siRNA and plated on fibronectin coverslips. (a) Cells were fixed at 96 hours post-transfection and assayed by immunofluorescence using TRITC phalloidin, coronin 1B, phospho-cofilin, and phospho-VASP antibodies. Nuclei were counterstained with DAPI. (b) Lysates were harvested 96 hours post-transfection and examined for expression of coronin 1B, cortactin (Millipore, Billerica, MA), Arp3 and α-actinin. Scale bar 10μm (JPEG 64 kb)
Supplementary Figure 3
Rsu1 depletion does not affect lumen formation in MCF10A and MCF10A infected clones. a. MCF10A cells were transfected with a control, Rsu1 and PINCH1 siRNA. At 72 hours post-transfection the cells were suspended in media containing 4% matrigel and seeded in matrigel coated wells of chamber slides. Cells were grown in MCF10A media for 14 days. MCF10A acinar structures cells were fixed at day 14 with 4% paraformaldehyde for 15 minutes at room temperature. Cells were rinsed once with PBS and permeabilized with 0.5% Triton in PBS for 10 min at 4ºC. After permeabilization, cells were washed, blocked and reacted with primary and secondary antibodies diluted in wash buffer + 10% goat serum. Rabbit anti-cleaved caspase 3 (Cell Signaling Technologies, Danvers, MA) was used for immune-fluorescence analysis. Alexa-Fluor conjugated antibodies were used as secondary antibodies. Chamber slides were mounted with ProLong Gold antifade reagent to detect DAPI. Images were captured with A Zeiss 710 Confocal Laser Scanning Microscope as Z-stacks with the number of slices recommended by the LSM software at a magnification of 40x. All channels were collected with the same optical unit setting. Data analysis was performed using the Zeiss LSM Image Browser. b. MCF10A puromycin selected cell lines were transfected with a control or Rsu1 siRNA. At 72 hours post-transfection the cells were suspended in media containing 4% matrigel and seeded in matrigel coated wells of chamber slides. MCF10A clones were grown in MCF10A media containing 1μg/ml puromycin for 14 days. At day 14 cells were fixed and processed as described above. Anti-Rsu1 rabbit polyclonal, rabbit anti-myc tag, mouse anti-E cadherin, and rabbit anti-cleaved caspase 3 were used for immunofluorescence analysis. Scale bar 10μm (JPEG 92 kb)
Supplementary Table 1
(DOCX 21 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gonzalez-Nieves, R., DeSantis, A.I. & Cutler, M.L. Rsu1 contributes to regulation of cell adhesion and spreading by PINCH1-dependent and - independent mechanisms. J. Cell Commun. Signal. 7, 279–293 (2013). https://doi.org/10.1007/s12079-013-0207-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-013-0207-5