Abstract
CCN2 plays a central role in the development and growth of mesenchymal tissue and promotes the regeneration of bone and cartilage in vivo. Of note, abundant CCN2 is contained in platelets, which is thought to play an important role in the tissue regeneration process. In this study, we initially pursued the possible origin of the CCN2 in platelets. First, we examined if the CCN2 in platelets was produced by megakaryocyte progenitors during differentiation. Unexpectedly, neither megakaryocytic CMK cells nor megakaryocytes that had differentiated from human haemopoietic stem cells in culture showed any detectable CCN2 gene expression or protein production. Together with the fact that no appreciable CCN2 was detected in megakaryocytes in vivo, these results suggest that megakaryocytes themselves do not produce CCN2. Next, we suspected that mesenchymal cells situated around megakaryocytes in the bone marrow were stimulated by the latter to produce CCN2, which was then taken up by platelets. To evaluate this hypothesis, we cultured human chondrocytic HCS-2/8 cells with medium conditioned by differentiating megakaryocyte cultures, and then monitored the production of CCN2 by the cells. As suspected, CCN2 production by HCS-2/8 was significantly enhanced by the conditioned medium. We further confirmed that human platelets were able to absorb/uptake exogenous CCN2 in vitro. These findings indicate that megakaryocytes secrete some unknown soluble factor(s) during differentiation, which factor stimulates the mesenchymal cells to produce CCN2 for uptake by the platelets. We also consider that, during bone growth, such thrombopoietic-mesenchymal interaction may contribute to the hypertrophic chondrocyte-specific accumulation of CCN2 that conducts endochondral ossification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CCN2/connective tissue growth factor (CTGF), which was initially regarded as a growth factor, has been now classified a member of the CCN family of multifunctional proteins (Lau and Lam 1999; Moussad and Brigstock 2000; Perbal 2004; Perbal and Takigawa 2005). The CCN family consists of 6 distinct members (CTGF/Fisp12/CCN2, Cyr61/Cef10/CCN1, Nov/CCN3, rCOP-1/WISP-1/CCN4, Elm-1/WISP-2/CCN5, and WISP-3/CCN6), each of which consists of four distinct structural modules, except for CCN5, which has three modules (Brigstock et al. 2003; Perbal and Takigawa 2005). CCN2 is known to exert multiple functions, and to play important roles in cartilage development and bone formation (Kubota and Takigawa 2007). Under physiological conditions, CCN2 is involved in embryonic development and differentiation of chondrocytes, osteoblasts, and vascular endothelial cells during endochondral ossification through all stages of this process. In fact, CCN2 null mice display severe skeletal abnormalities and die soon after birth because of impaired endochondral bone formation (Ivkovic et al. 2003). Under pathological conditions, CCN2 is associated with fibrosis (Ahmed and Øie 2007), tumor angiogenesis (Shimo et al. 1999), cell migration (Ono et al. 2008), and the regeneration process of wounded tissues (Nakata et al. 2002; Kanyama et al. 2003; Shi-Wen et al. 2008). Our recent reports have revealed that CCN2 was capable of accelerating harmonized regeneration of defective cartilage and bone in animal models (Nishida et al. 2004; Kikuchi et al. 2008). Consistent with its regeneration potential, CCN2 is widely known to be stored exclusively in normal platelets (Kubota et al. 2004). Platelets, also referred to as thrombocytes, are anucleate fragments of megakaryocytes and play a central role in clotting (Jennings 2009). Platelets are produced by mature megakaryocytes, which are differentiated from haemopoietic progenitors in the bone marrow after birth, as are the other blood cells. These small “cells” without nuclei contain many bioactive substances involved in cell adhesion and activation, as well as in coagulation (Kaushansky 2008). Therefore, platelets play a principal role not only in the coagulation process but also in wound healing. It should be noted that, in recent years, platelet-rich plasma (PRP) has been attracting the attention of clinicians and researchers as a new approach for connective tissue engineering (Akeda et al. 2006; Agis et al. 2009; Simman et al. 2008). The regeneration potential of platelets is due to the inclusion in them of a number of growth factors, such as platelet-derived growth factor (PDGF), transforming growth factor (TGF) β1 and 2, and insulin-like growth factor-1 (IGF-1; Weibrich et al. 2002; Cicha et al. 2004; van den Dolder et al. 2006; Barrientos et al. 2008). Concerning CCN2, previous reports described that platelets contain much more CCN2 protein than other normal cells or tissues, indicating its putative role in the early stage of tissue regeneration (Kubota et al. 2004; Cicha et al. 2004). In fact, platelets release a vast amount of CCN2 upon activation. These findings suggest CCN2 to be an indispensable component of platelets as tissue regenerators after local injury. However, the origin of CCN2 in platelets is currently unknown. In other words, how such a secreted protein with a signal peptide can be stored inside of the platelets still remains to be clarified.
In this study, we investigated the origin of CCN2 accumulated in platelets, beginning from the analysis of the platelet producer, i.e., the megakaryocyte. In this process, quite interesting findings indicating an interaction between megakaryocytic and chondrocytic cells in the bone marrow microenvironment were made. Here, we describe a possible thrombopoietic-mesenchymal interaction that is crucial for both the formation of platelets and skeletal development.
Materials and methods
Isolation of haemopoietic stem cells and in vitro differentiation of megakaryocyte progenitors
Human CD34-positive cells were immunomagnetically isolated from cord-blood mononuclear cells by an established procedure using a MACS CD34 Progenitor Cell Isolation kit (Miltenyi Biotech). The cord-blood cells were obtained from Keihan Cord Blood Bank with the approval of an external ethics committee. In vitro differentiation was induced by culturing these CD34-positive cells in RPMI1640 supplemented with 20% human serum (blood type: AB) and 20 ng/ml of thrombopoietin (Peprotech). At 0, 3, and 10 days after the initiation of the cell cultures, the cells and conditioned media were separately harvested by centrifugation. Proper differentiation to the megakaryocytic lineage was evaluated by a flow cytometer analysis with a PE-labeled anti-CD41 (Immunotech, BD Bioscience) or FITC-labeled anti-CD34 monoclonal antibody (Immunotech, Beckmann-Coulter).
Cell lines
Two human cell lines were employed. A human megakaryocytic cell line, CMK (Yasui et al. 2005) was maintained in RPMI1640 containing 10% fetal bovine serum (FBS). A human chondrocytic cell line, HCS-2/8, was established from a human chondrosarcoma (Takigawa et al. 1989) and was cultured in Dulbecco’s modified minimal essential medium (D-MEM) containing 10% FBS.
RNA extraction and RT-PCR
Total RNA was extracted by the acid-guanidinium phenol-chloroform method with Trizol reagent, according to the manufacturer’s protocol. Reverse transcription was carried out by using avian myeloblastosis virus (AMV) reverse transcriptase with 200 ng of each total RNA at 42°C for 30 min. Subsequent PCR cycles consisted of an initial denaturation at 94°C for 5 min and 40 cycles of amplification reaction at 94°C for 20 sec, 51°C for 20 sec and 72°C for 20 sec with Taq polymerase (NEB). PCR products were analyzed by 1.5% agarose gel electrophoresis and ethidium bromide staining.
Indirect immunofluorescence analysis of platelets
Human platelets were smeared onto a slice glass and immediately air-dried. Thereafter, they were fixed in 3.5% formaldehyde in phosphate-buffered saline (PBS) and subsequently incubated with a polyclonal antibody against human CCN2 (anti-CTGFw; Kubota et al. 2004). Immunofluorescence analysis was performed essentially as described previously (Kubota et al. 2000).
Preparation of platelets and CCN2-absorption analysis
Human platelets were concentrated from human peripheral blood from 2 healthy volunteers. Initially, leukocytes and erythrocytes were removed by centrifugation at ×130 g for 15 min. Thereafter, the platelets were concentrated by another centrifugation at ×900 g for 10 min. The resultant plasma contained 4 × 105 platelets/µl. The platelets (2 × 107) were then exposed to 200 ng/ml of recombinant CCN2 in 100 µl of 50% plasma in Dulbecco’s phosphate-buffered saline (PBS) for the desired time periods. Immediately after the addition of 1 ml of PBS for wash, the platelets were pelleted by centrifugation at ×2,000 g for 7 min and lysed in 50 µl of a 1 × sodium dodecyl-sulfate (SDS) sample buffer containing 2-mercaptoethanol. Equal volumes of each sample were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting analysis.
CCN2 induction assay with HCS-2/8 cells
HCS-2/8 cells were utilized to estimate the effect of soluble factors in medium conditioned by megakaryocyte progenitors on the CCN2 production by the mesenchymal cells. The mesenchymal HCS-2/8cells were grown in D-MEM supplemented with 10% FBS and seeded at a cell density of 5 × 105/35-mm in tissue culture dishes. Thereafter, serum-free D-MEM mixed with a quarter volume of each conditioned medium from megakaryocyte in vitro differentiation cultures was added to replace the growth medium. Twelve hours after the medium change, the culture supernatant was collected and subjected to ELISA for the quantification of CCN2 secreted from the HCS-2/8 cells.
CMK-HCS-2/8 co-culture analysis
HCS-2/8 cells were seeded at a density of 5 × 105 cells per well in a 6-well cell culture plate and cultured overnight. Thereafter, CMK cells (1.5 × 106) in RPMI1640 containing 10% FBS were added to replace the tissue culture medium. After 0, 6, and 24 h, the tissue culture supernatant and floating cells were separately recollected by centrifugatioon. More than 90% of the recollected cells were confirmed to be CD41+ via FACS analysis. CMK cell lysates were prepared by adding 1 × 106 CMK cells to 1 ml of RIPA buffer (50 mM Tris-HCl, pH 8.0, containing 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, and 0.1% SDS). CCN2 in the tissue culture supernatant and cell lysate was quantified by ELISA.
Western blotting analysis
SDS-PAGE and Western transfer of proteins were performed as described previously (Kubota et al. 2004). Briefly, proteins in 20 µl of platelet lysate described in a previous subsection were separated by SDS-PAGE (in a 12% acrylamide gel), and transferred onto a polyvinylidene difluoride (PVDF) membrane (Amersham Bioscience). After having been blocked with 5% skim milk in Tris-buffered saline (TBS), the membrane was incubated at 4°C for 24 h with 1:350-fold-diluted mouse monoclonal antibody against CCN2 von Willebrand factor type C repeat (VWC) module (Minato et al. 2004) or 1:1,000-fold diluted mouse monoclonal anti-actin antibody (SIGMA St. Louis, MO, USA). After extensive washes, it was then incubated with 1:5,000-fold diluted anti-mouse IgG horseradish peroxidase (HRP) conjugate (GE Healthcare). The blot was visualized by use of an enhanced chemiluminescence (ECL) analysis system (GE Healthcare).
Enzyme-linked immunosorbent assay (ELISA)
CCN2 was quantified by using a sandwich ELISA system with two anti-human CCN2 monoclonal antibodies (MAb 8-64 and 8-86; mouse IgG1) provided by Nichirei Corp. (Tokyo, Japan), as described previously (Kubota et al. 2004). In brief, samples were applied to ELISA strips coated with MAb 8-64 and incubated for 2 h. After six cycles of washing, horseradish peroxidase-conjugated MAb 8-86 was added, and the strips were incubated for 1 h. The signals were developed via an enzymatic reaction with tetramethylbenzidine.
Immunohistochemistry
Immunohistochemical staining was performed as described previously (Moritani et al. 2003). Briefly, paraffin-embedded sections from 6-week-old mice were deparaffinized, blocked and incubated with an anti-CCN2 serum reactive with murine CCN2 at a dilution of 1: 200. After the reaction with horseradish peroxidase-conjugated secondary antibody, the signals were developed by use of 3′-diaminobenzidine. Finally, the samples were counterstained with hematoxylin.
Results
Evaluation of the CCN2 gene expression and protein production by megakaryocytic CMK cells
Since platelets are directly produced by megakaryocytes, we first suspected megakaryocytes as the source of CCN2 encapsulated in platelets (Fig. 1a). As an initial step, we employed the CMK cell line, a human cell line retaining the megakaryocytic phenotype, such as CD41 expression, for the evaluation of CCN production. However, neither CCN2 mRNA expression (data not shown) nor CCN2 production/secretion (Fig. 1b) was detectable under regular cell culture conditions.
a Three possible pathways to supply CCN2 to platelets. I: CD41+ megakaryocyte progenitor cells produce CCN2, which is then taken up by the platelets. II: Megakaryocytes themselves produce CCN2 that is directly encapsulated upon platelet formation. III: CCN2 is produced by the cells of another lineage, such as mesenchymal cells, in the same microenvironment and is taken up by platelets. Abbreviations: Meg megakaryocyte; Pt platelet. b No CCN2 production from the human megakaryocytic cell line CMK. CD41+ CMK cells were cultured for 7 days without changing the medium, and CCN2 protein in the culture supernatant was analyzed by ELISA. As a positive control, the CCN2 concentration in the culture supernatant of HCS-2/8 cells stimulated with 2.5% FBS for 12 h is presented. The dotted line indicates the background level obtained with the medium only (18.2 ng/ml). Data were obtained from 2 independent sets of samples
Evaluation of the CCN2 gene expression and protein production by differentiating megakaryocytic progenitors in vitro
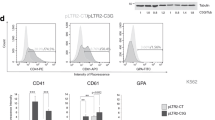
Since CMK is an established immortal cell line, it may not precisely retain the complete megakaryocytic phenotype. Therefore, we subsequently isolated CD34-positive haemopoietic stem cells from human cord blood leukocytes by cell sorting; and cytodifferentiation towards megakaryocyte progenitors was induced by incubation with recombinant thrombopoietin. According to the result of flow cytometric analysis, those cells initially showed morphological changes such as increased cell size, which was represented by the forward scatter values, 3 days after initiating the induction of differentiation. Thereafter, demonstration of CD41 molecules was clearly observed 10 days after the start of the cell cultures (Fig. 2a). These findings indicate that the isolated cells were mostly composed of haemopoietic stem cells that could differentiate into megakaryocyte progenitors in vitro. Under the same culture conditions, total RNA was isolated and subjected to PCR analysis for the detection of CCN2 mRNA. However, no CCN2 mRNA was detectable in the cells in the differentiating cultures at either 0, 3 or 10 days after the beginning of the cultures; whereas β-actin mRNA was definitely detected at all times (Fig. 2b). Consistent with this result, no CCN2 protein was detected in the cell culture supernatant, either (Fig. 2c). Therefore, we concluded that, during the differentiation in vitro, CCN2 was not produced by megakaryocyte progenitors. In addition, since terminal differentiation to mature megakaryocytes producing platelets was not inducible in vitro, we performed immunohistochemical staining to detect CCN2 in mature megakaryocytes in mouse bone marrow. Consequently, we could detect no positive signal representing CCN2 production (data not shown; Cicha et al. 2004).
Analysis of CCN2 gene expression and protein production during the course of megakaryocytic differentiation. Human cord blood CD34+ cells were caused by thrombopoietin to differentiate in vitro toward the megakaryocyte lineage. The cells were sampled at the indicated time points and then analyzed by FACS for cell morphology and demonstration of cell-surface antigens (a), by RT-PCR for CCN2 expression (b), and by ELISA for CCN2 production (c). Abbreviations SSC, FSC, PE, and FITC in panel A denote side scatter, forward scatter, propidium iodide, and fluorescein isothiocyanate, respectively. Total RNA from HCS-2/8 cells was utilized as a positive control in panel b. Similar experiments with peripheral blood-derived haemopoietic stem cells yielded comparable results (data not shown)
Effect of the culture medium conditioned by differentiating megakaryocyte progenitors on the CCN2 production by mesenchymal cells
Platelets are known to take up a variety of molecules via endocytosis. Therefore, we addressed the possibility that megakaryocyte progenitors may force other cells in the same microenvironment to secrete CCN2, which is subsequently taken up and accumulated in the nascent platelets. Megakaryocytes mature in the bone marrow, in which a wide variety of mesenchymal cells reside together with the haemopoietic cells. Among these mesenchymal cells, growth plate chondrocytes facing the bone marrow are known to produce a vast amount of CCN2 in vivo; whereas other types of mesenchymal cells, such as osteoblasts and mesenchymal stem cells, produce only limited amounts. In order to examine if megakaryocytes contribute to the induction of CCN2 production by chondrocytes, we evaluated the effect of the conditioned medium from megakaryocyte cultures on the production of CCN2 by chondrocytic cells (Fig. 3a). HCS-2/8 cells, which were known to retain the chondrocytic phenotype, were employed. As a result, the amount of CCN2 secreted by HCS-2/8 cells was remarkably increased by the addition of the conditioned medium from differentiating megakaryocyte cultures taken at day 3 (Fig. 3b). These data indicate that megakaryocyte progenitors produce some unknown soluble factor(s) that targets surrounding mesenchymal cells, including prehypertrophic/hypertrophic chondrocytes, and forces them to produce CCN2.
Promotion of CCN2 production from chondrocytic HCS-2/8 cells by a soluble factor produced by megakaryocyte progenitors. a Experimental strategy. The conditioned medium was sampled at days 0, 3, and 10 from human CD34-positive cell cultures differentiating toward megakaryocytes. HCS-2/8 cells were maintained in the presence of each conditioned medium (20% in serum free D-MEM) for 12 h, and the cell culture supernatant was then subjected to ELISA for the quantification of the secreted CCN2. b Production of CCN2 from HCS-2/8 cells stimulated by the culture supernatants. The data show the concentration of CCN2 accumulated in the HCS2/8 cell culture supernatant during 12 h after adding the conditioned medium from the megakaryocyte progenitor cultures. Experiments were repeated twice, and comparable results were obtained
Effect of the culture medium conditioned by CMK cells on the CCN2 production by mesenchymal cells
According to the results shown in Fig. 3, the ability of differentiating megakaryocyte progenitors to stimulate CCN2 production from HCS-2/8 cells diminished at a later stage of differentiation. In order to further confirm this finding, we evaluated the corresponding ability of CMK cells. As shown in Fig. 4a, CMK cells were confirmed to retain the phenotype of megakaryocyte progenitors at a late stage of differentiation, as represented by a high level of CD41 without CD34. As expected, the result of the CCN2 induction assay revealed no enhancement of CCN2 production from HCS-2/8 cells by the CMK conditioned medium (Fig. 4b). Therefore, these data indicate that the factor stimulating CCN2 production was released predominantly by the megakaryocyte progenitors at relatively early stages of differentiation.
No effect of the conditioned medium from CMK cells on the CCN2 production from HCS-2/8 cells. a CMK cells show the phenotype of megakaryocyte progenitors at a late differentiation stage. The presence of CD41 (abscissa) and CD34 (ordinate) on CMK cells was examined, with the results plotted in a 2-dimensional representation. Isotype control is also shown. The percentage of each population is indicated in each section as well. b Effect of the conditioned medium from CMK cells maintained for 3 days on the CCN2 production from HCS-2/8 cells. The relatively high background (day 0) may be ascribed to the involvement of 10% FBS in the conditioned medium
Localization of CCN2 in platelets
Although the inclusion of CCN2 in platelets has been clearly demonstrated, its subcellular localization therein has not been analyzed. Thus, we carried out immunofluorescence analysis to investigate the mode of CCN2 distribution in platelets. As a result, CCN2 was not distributed evenly in the cytoplasm, but had accumulated in granular structures (Fig. 5a).
a Distribution of CCN2 in platelets. CCN2 in human platelets was visualized by immunofluorescence analysis and was viewed at a magnification of ×400. CCN2 is found in granular structures therein. b Absorption/incorporation of exogenous CCN2 to/by human platelets. After the addition of CCN2, human platelets were collected and subjected to Western blotting for the detection of CCN2. Western blotting against actin was also performed as an internal control. The intensity of the signals at 38 kDa, representing full-length CCN2, increased with time up to 40 min after the addition of CCN2. The minor bands are anticipated to be proteolytic N-terminal fragment of CCN2. Evaluation was performed with two samples from independent donors, and similar results were obtained
Absorption/uptake of extracellular CCN2 by platelets
The fact that CCN2 was found in platelet granules suggests that CCN2 might have been endosomally acquired and accumulated in the granules until released. Thus, we next tested to see if normal platelets with CCN2 were still capable of taking up extracellular CCN2. Platelets were concentrated from human blood, and CCN2 was exogenously added to the platelet suspension. Thereafter, the platelets were collected; and the platelet-associated CCN2 was then detected by Western blotting. After the addition of the exogenous CCN2 to platelets, the quantity of CCN2 associated with platelets increased with time (Fig. 5b). Although it is not clear whether the platelets had incorporated the CCN2 by endocytosis or had simply adsorbed it, the observed continuous increase in CCN2 up to 40 min may represent intracellular uptake of CCN2 by platelets. It should be noted here that a number of proteins have been shown to be taken up by endocytosis into platelets (Escolar et al. 2008).
No significant transfer of CCN2 from mesenchymal HCS-2/8 cells to CD41-positive CMK cells
In addition to the direct uptake by platelets, another possible pathway to package exogenous CCN2 in platelets is the endocytotic incorporation by megakaryocytic progenitors. To evaluate this possibility, we co-cultured CMK cells with HCS-2/8 cells (Fig. 6a), and then quantitatively analyzed CCN2 in CMK cells after conducting a time-course experiment. However, although CCN2 is efficiently produced by HCS-2/8 cells, no incorporation of CCN2 by CMK cells was observed (Fig. 6b). Therefore, transfer of CCN2 from mesenchymal cells to megakaryocytic progenitors cells may not occur, even under the direct cell-to-cell contact between them.
Evaluation of the intercellular transfer of CCN2 from HCS-2/8 to CMK cells. a Experimental strategy. CMK cells were seeded onto HCS-2/8 cells that had been attached to tissue culture wells and allowed to make cell-to-cell contact. b No transfer of CCN2 into CMK cells. After the indicated intervals, CCN2 in CMK cell lysates and co-culture media was quantified by ELISA
Abundant CCN2 found in hypertrophic chondrocytes
It is known that CCN2 is mainly produced by hypertrophic chondrocytes facing, or adjacent to bone marrow in the growth plate (Takigawa et al. 2003), which is consistent with our present findings. To confirm this point, we reevaluated the distribution of CCN2 in the mouse growth plate and found that CCN2 was abundantly produced by late hypertrophic chondrocytes near the bone marrow, whereas a relatively limited amount was detected in the inner side (Fig. 7a). These results suggest a possible haemopoietic-mesenchymal interaction that enables the enhanced CCN2 production by hypertrophic chondrocytes.
a Immunostaining of CCN2 produced by chondrocytes in the hypertrophic layer of mouse growth plate near the bone marrow. Note that cells on the bone marrow side accumulate more CCN2 than those on the inner side. b Schematic representation of possible thrombopoietic-mesenchymal interaction around the growth plate-bone marrow boundary. We hypothesize that megakaryocyte progenitors secrete some unknown soluble factor during differentiation, which factor stimulates the chondrocytes facing the bone marrow to produce CCN2. During bone growth, this interaction contributes to the accumulation of CCN2, not only in platelets, but also in the hypertrophic chondrocytes that conduct proper endochondral ossification. Abbreviations: R resting chondrocyte; G growing chondrocyte; Pre-H pre-hypertrophic chondrocyte; H hypertrophic chondrocyte; Meg megakaryocyte
Discussion
In the present study, we first pursued the origin of CCN2 in platelets. Initially we suspected megakaryocytes to be the direct source; however, we consequently detected no CCN2 mRNA or protein in megakaryocytes or in their progenitors, including the CMK cell line. Next, we investigated the mesenchymal cells around platelets to examine if CCN2 could be supplied by those cells following interaction with megakaryocyte progenitors. As a result, we uncovered a quite interesting interaction between megakaryocyte progenitors and mesenchymal cells in vitro. Recently, the importance of interactions between haemopoietic and mesenchymal cells has been widely recognized. Indeed, the regulation of self-renewal and differentiation of haemopoietic stem cells (HSC) requires a specific microenvironment of surrounding cells known as the niche, in which osteoblasts and HSCs tightly interact via angiopoietin-1 and Tie-2 (Arai et al. 2004; Calvi et al. 2003). It is widely known that megakaryocyte progenitors do not undergo final maturation to produce platelets in vitro. Therefore, megakaryocytes also need a certain interaction(s) with non-haemopoietic cells, including mesenchymal cells, in order to mature and produce functional platelets. Here, we found one such interaction between megakaryocytes and mesenchymal cells (Fig. 5), which supplies CCN2 to platelets to accomplish their maturation, since CCN2 is an important component of platelets for their tissue-repair function. The cell-surface molecules that mediate the incorporation of CCN2 into platelets are unclear. However, one of the CCN2 receptors, low-density lipoprotein receptor-related protein 1 (LRP1), is known to function as an endocytotic receptor (Kawata et al. 2006). Moreover, direct interaction of CCN2 with integrin αIIbβ3, which was shown to mediate endocytosis of fibrinogen into platelets (Handagama et al. 1993; Jedsadayanmata et al. 1999), is clearly a possibility. Investigation of the interaction of CCN2 with these molecules will eventually clarify if/how CCN2 is incorporated by endocytosis.
The role of CCN2 in wound healing and tissue regeneration has been widely recognized. The CCN2 gene is expressed in the process of tissue regeneration that occurs in the wound healing process and bone fracture repair. Furthermore, the utility of CCN2 in tissue regenerative therapeutics has been established. Recently in many fields, the clinical application of platelet-rich plasma (PRP) has been frequently reported; and PRP has significant effects on soft and hard tissue regeneration, which are comparable to the effect of cytokine treatment (Mirabet et al. 2008; Simman et al. 2008). Additionally, it should be noted that PRP is quite safe and is relatively not expensive. Obviously, CCN2 abundantly included in platelets may contribute to the regeneration potential of platelets. As such, proper encapsulation of CCN2 is a critical step for platelets to be fully functional as a key player in wound healing.
In the growth plate, CCN2 is abundantly produced by a limited population of chondrocytes facing the bone marrow, which cells are called hypertrophic/prehypertrophic chondrocytes. This specific CCN2 production in hypertrophic/prehypertrophic chondrocytes is widely known and is necessary for the proper endochondral ossification leading to the normal growth of long bones (Fig. 7a; Kubota and Takigawa 2007). Nevertheless, the mechanism as to how the production of CCN2 is strongly induced only in hypertrophic/prehypertrophic chondrocytes around the bone marrow is still unclear. In this context, our present data suggest for the first time the involvement of thrombopoietic-mesenchymal interaction in the mechanism that causes hypertrophic/prehypertrophic chondrocyte-specific accumulation of CCN2 to conduct endochondral ossification. Thus, this haemopoietic-mesenchymal cell interaction may be critical not only for platelet maturation but also in the endochondral ossification process (Fig. 7b).
However, a number of issues still remain to be investigated. First of all, the soluble factor that is produced by megakaryocytic progenitors and induces CCN2 production has not been specified yet. We initially suspected TGF-β as such a molecule and tried to detect TGF-β mRNA in megakaryocyte progenitors, because this factor is known to promote CCN2 production in a variety of the cells. However, neither mRNA for TGF-β1 nor TGF-β2 was detected (data not shown). As a second issue, chondrocytes are available during the growth period as suppliers of CCN2. Then, after the closure of the growth plate, which mesenchymal cells would produce CCN2 for platelets? One such candidate is the mesenchymal stromal cell, but no data to support this hypothesis are available yet. So far as platelets are produced through the life, some other major CCN2 producer(s) than the hypertrophic chondrocytes must be found in adult bone marrow. Identification of the soluble factor that provokes CCN2 production and evaluation of the responsiveness to this factor by other types of mesenchymal cells are currently underway.
References
Agis H, Kandler B, Fischer MB, Watzek G, Gruber R (2009) Activated platelets increase fibrinolysis of mesenchymal progenitor cells. J Orthop Res 27:972–980
Ahmed MS, Øie E (2007) Induction of pulmonary connective tissue growth factor in heart failure is associated with pulmonary parenchymal and vascular remodeling. Cardiovasc Res 74:323–333
Akeda K, An HS, Okuma M, Attawia M, Miyamoto K, Thonar EJ, Lenz ME, Sah RL, Masuda K (2006) Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthr Cartil 14:1272–1280
Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T (2004) Tie2/Angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 23:149–161
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16:585–601
Brigstock DR, Goldschmeding R, Katsube K, Lam SCT, Lau LF, Lyons K, Naus C, Perbal B, Riser B, Takigawa M, Yeger H (2003) Proposal for a unified CCN nomenclature. Mol Pathol 56:127–128
Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR (2003) Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425:841–846
Cicha I, Garlichs CD, Daniel WG, Goppelt-Struebe M (2004) Activated human platelets release connective tissue growth factor. Thromb Haemost 91:755–760
Escolar G, Lopez-Vilchez I, Diaz-Ricart M, White JG, Galan AM (2008) Internalization of tissue factor by platelets. Thromb Res 122:S37–41
Handagama P, Scarborough RM, Shuman MA, Bainton DF (1993) Endocytosis of fibrinogen into megakaryocyte and platelet alpha-granules is mediated by alpha IIb beta 3 (glycoprotein IIb-IIIa). Blood 82:135–138
Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM (2003) Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 130:2779–2791
Jedsadayanmata A, Chen CC, Kireeva ML, Lau LF, Lam SC (1999) Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin alpha(IIb)beta(3). J Biol Chem 274:24321–24327
Jennings LK (2009) Role of platelets in atherothrombosis. Am J Cardiol 103:4A–10A
Kanyama M, Kuboki T, Akiyama K, Nawachi K, Miyauchi FM, Yatani H, Kubota S, Nakanishi T, Takigawa M (2003) Connective tissue growth factor expressed in rat alveolar bone regeneration sites after tooth extraction. Arch Oral Biol 48:723–730
Kaushansky K (2008) Historical review: megakaryopoiesis and thrombopoiesis. Blood 111:981–986
Kawata K, Eguchi T, Kubota S, Kawaki H, Oka M, Minagi S, Takigawa M (2006) Possible role of LRP1, a CCN2 receptor, in chondrocytes. Biochem Biophys Res Commun 345:552–559
Kikuchi T, Kubota S, Asaumi K, Kawaki H, Nishida T, Kawata K, Mitani S, Tabata Y, Ozaki T, Takigawa M (2008) Promotion of bone regeneration by CCN2 incorporated into gelatin hydrogel. Tissue Eng Part A. 14:1089–1098
Kubota S, Takigawa M (2007) Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol 257:1–41
Kubota S, Hattori T, Shimo T, Nakanishi T, Takigawa M (2000) Novel intracellular effects of human connective tissue growth factor expressed in Cos-7 cells. FEBS Lett 474:58–62
Kubota S, Kawata K, Yanagita T, Doi H, Kitoh T, Takigawa M (2004) Abundant retention and release of connective tissue growth factor (CTGF/CCN2) by platelets. J Biochem 136:279–282
Lau LF, Lam SCT (1999) The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res 248:44–57
Minato M, Kubota S, Kawaki H, Nishida T, Miyauchi A, Hanagata H, Nakanishi T, Takano-Yamamoto T, Takigawa M (2004) Module-specific antibodies against human connective tissue growth factor: Utility for structural and functional analysis of the factor as related to chondrocytes. J Biochem 135:347–354
Mirabet V, Solves P, Miñana MD, Encabo A, Carbonell-Uberos F, Blanquer A, Roig R (2008) Human platelet lysate enhances the proliferative activity of cultured human fibroblast-like cells from different tissues. Cell Tissue Bank 9:1–10
Moritani NH, Kubota S, Eguchi T, Fukunaga T, Yamashiro T, Takano-Yamamoto T, Tahara H, Ohyama K, Sugahara T, Takigawa M (2003) Interaction of AP-1 and the ctgf gene: a possible driver of chondrocyte hypertrophy in growth cartilage. J Bone Miner Metab 21:205–210
Moussad E, Brigstock DR (2000) Connective tissue growth factor: what’s in a name? Mol Genet Metab 71:276–292
Nakata E, Nakanishi T, Kawai A, Asaumi K, Yamaai T, Asano M, Nishida T, Mitani S, Inoue H, Takigawa M (2002) Expression of connective tissue growth factor/hypertrophic chondrocyte-specific gene product 24 (CTGF/Hcs24) during fracture healing. Bone 31:441–447
Nishida T, Kubota S, Kojima S, Kuboki T, Nakao K, Kushibiki T, Tabata Y, Takigawa M (2004) Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor). J. Bone Miner Res 19:1308–1319
Ono M, Kubota S, Fujisawa T, Sonoyama W, Kawaki H, Akiyama K, Shimono K, Oshima M, Nishida T, Yoshida Y, Suzuki K, Takigawa M, Kuboki T (2008) Promotion of hydroxyapatite-associated, stem cell-based bone regeneration by CCN2. Cell Transplant 17:231–240
Perbal B (2004) CCN proteins: multifunctional signalling regulators. Lancet 363:62–64
Perbal B, Takigawa M (2005) CCN protein—a new family of cell growth and differentiation regulators. Imperial College, London, pp 1–311
Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, Takigawa M (1999) Connective tissue growth factor induces the proliferation, migration and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem 126:137–145
Shi-Wen X, Leask A, Abraham D (2008) Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev 19:133–144
Simman R, Hoffmann A, Bohinc RJ, Peterson WC, Russ AJ (2008) Role of platelet-rich plasma in acceleration of bone fracture healing. Ann Plast Surg 61:337–344
Takigawa M, Tajima K, Pan HO, Enomoto M, Kinoshita A, Suzuki F, Takano Y, Mori Y (1989) Establishment of a clonal human chondrosarcoma cell line with cartilage phenotypes. Cancer Res 49(3):996–4002
Takigawa M, Nakanishi T, Kubota S, Nishida T (2003) Role of CTGF/HCS24/ecogenin in skeletal growth control. J Cell Physiol 194:256–266
van den Dolder J, Mooren R, Vloon AP, Stoelinga PJ, Jansen JA (2006) Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng 12:3067–3073
Weibrich G, Kleis WK, Hafner G, Hitzler WE (2002) Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg 30:97–102
Yasui K, Furuta RA, Matsumoto K, Tani Y, Fujisawa J (2005) HIV-1-derived self-inactivating lentivirus vector induces megakaryocyte lineage-specific gene expression. Microbes Infect 7:240–247
Acknowledgments
This study was supported by grants from the program Grants-in-aid for Scientific Research (S) [to M.T.], and (C) [to S.K.] from the Japan Society for the Promotion of Science. We thank Drs. Takashi Nishida, Shinsuke Itoh, and Takako Hattori for their valuable discussions.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Concise summary
The mechanisms as to how CCN2 is accumulated in platelets and how it is abundantly produced by hypertrophic/prehypertrophic chondrocytes have remained unclear. Here, we describe an interaction between megakaryocytic and chondrocytic cells, which may supply CCN2 to both platelets and hypertrophic chondrocytes.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Sumiyoshi, K., Kubota, S., Furuta, R.A. et al. Thrombopoietic-mesenchymal interaction that may facilitate both endochondral ossification and platelet maturation via CCN2. J. Cell Commun. Signal. 4, 5–14 (2010). https://doi.org/10.1007/s12079-009-0067-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-009-0067-1