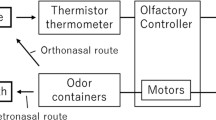
Abstract
Perceived intensities of continuously available orthonasal and retronasal vapor-phase stimuli during natural breathing for approximately the first minute of smelling were not known in detail. To clarify this, stimuli presented continuously in vapor phase orthonasally or retronasally during natural breathing were time–intensity tracked over 55 s by 19 participants, with tracked intensities measured every 100 ms. Before the tracking session, participants individually matched concentrations of anise, coffee, peppermint, and strawberry flavors to the intensity of a fifth stimulus, orange. These were their tracked concentrations. During the intensity tracking sessions, real-time visual feedback of judged intensity was provided on a computer display. Participants controlled the vertical position of the display (intensity) while the horizontal position (time) advanced at a constant rate under program control, creating an image of intensity with respect to time. It was found that during natural breathing, retronasal-tracked intensity increased to maximum at ∼8 s, but was approximately half of orthonasal tracked maximum intensity, reached after ∼11 s. Within the 55-s tracking period, retronasal and orthonasal maxima exceeded their initial and final intensities. Final intensities at 55 s were approximately half of the maximum intensities. These data indicate, with high-resolution tracking during natural breathing of continuously presented vapor-phase stimuli, that intensity of smelling increases to a maximum after initial intensity is perceived, and that smelled intensity then declines slowly during approximately the first minute of smelling.



Similar content being viewed by others
References
Boeckh J, Kaissling KE, Schneider D (1965) Insect olfactory receptors. Cold Spring Harbor Symposia on Quantitative Biology 30 (Sensory Receptors): 263–280
Cain WS (1974) Perception of odor intensity and the time-course of olfactory adaption. ASHRAE Trans 80:53–75
Cain WS (1976) Olfaction and the common chemical sense: some psychophysical contrasts. Sensory Process 1:57–67
Cain WS (1990) Perceptual characteristics of nasal irritation. In: Green BG, Mason JR, Kare MR (eds) Chemical senses, vol 2, Irritation. Decker, New York, pp 43–60
Cain WS, Krause RJ (1979) Olfactory testing: rules for odor testing. Neurol Res 1:1–9
Cain WS, Murphy CL (1980) Interaction between chemoreceptive modalities of odour and irritation. Nature 284:255–257
Cain WS, Leaderer BP, Cannon L, Tosun T, Ismaila H (1987) Odorization of inert gas for occupational safety: psychophysical considerations. Am Ind Hyg Assoc J 48(1):47–55
Cain WS, Goodspeed RB, Gent JF, Leonard G (1988) Evaluation of olfactory dysfunctions in the Connecticut Chemosensory Clinical Research Center. Laryngoscope 98:83–88
Caprio J, Byrd RP Jr (1984) Electrophysiological evidence for acidic, basic, and neutral amino acid olfactory receptor sites in the catfish. J Gen Physiol 84:403–422
Chalé-Rush A, Burgess JR, Mattes RD (2007) Multiple routes of chemosensitivity to free fatty acids in humans. Am J Physiol Gastrointestinal Liver Physiol 292:1206–1212
Chen V, Halpern BP (2008) Retronasal but not oral-cavity-only identification of “purely olfactory” odorants. Chem Senses 33:107–118
Clark CC, Lawless HT (1994) Limiting response alternatives in time-intensity scaling: an examination of the halo-dumping effect. Chem Senses 19:583–594
Dalton P (2002) Olfaction. Stevens’ Handbook of experimental psychology, 3rd ed, vol 1. In: Yantis S, Pashler H (eds) Sensation and perception. Wiley, New York, pp 691–746
Dalton P, Wysocki CJ, Brody MJ, Lawley HJ (1997) The influence of cognitive bias on the perceived odor, irritation and health symptoms from chemical exposure. Int Arch Occup Environ Health 69:407–417
Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD (1978) Intranasal trigeminal stimulation from odorous volatiles: psychometric responses. Physiol Behav 20:175–185
Dragich AM, Halpern BP (2008) An oral cavity component in retronasal smelling of natural extracts. Physiol Behav 93:521–528
Ferguson SSG, Caron MG (1998) G protein-coupled receptor adaptation mechanisms. Cell Devel Biol 9:119–127
Frasnelli J, Heilmann S, Hummel T (2004) Responsiveness of human nasal mucosa to trigeminal stimuli depends on the site of stimulation. Neurosci Letters 362:65–69
Friedrich RW, Laurent G (2001) Dynamic optimization of odor representation by slow temporal patterning of mitral cell activity. Science 291:889–894
Geffen MN, Broome BM, Laurent G, Meister M (2009) Neural encoding of rapidly fluctuating odors. Neuron 61:570–586
Golaszewski R, Sims CA, O'Keefe SF, Braddock RJ, Littell RC (1998) Sensory attributes and volatile components of stored strawberry juice. J Food Sci 63:734–738
Halpern BP (2004) When are oral cavity odorants available for retronasal olfaction? In: Deibler KD, Delwiche J (eds) Handbook of flavor characterization: sensory analysis, chemistry, and physiology. Marcel Dekker, New York, pp 51–63
Halpern BP (2008) Mechanisms and consequences of retronasal smelling: computational fluid dynamic observations and psychophysical measures. ChemoSense 10(3):1–8
Hearst E (1988) Fundamentals of learning and conditioning. In: Atkinson RC, Herrnstein RJ, Lindzey G, Luce D (eds) Stevens’ handbook of experimental psychology, vol 2, 2nd edn, Learning and cognition. Wiley, New York, pp 3–109
Heilmann S, Hummel T (2004) A new method for comparing orthonasal and retronasal olfaction. Behav Neurosci 118:412–419
Homma Y, Komatsu H, Higashi N, Shikata H (2003) Analysis of food flavors by gas chromatography-retronasal olfactometry. Chem Senses 28:A28
Hummel T, Knecht M, Kobal G (1996) Peripherally obtained electrophysiological responses to olfactory stimulation in man: electro-olfactograms exhibit a smaller degree of desensitization compared with subjective intensity estimates. Brain Research 717:160–164
Kosinski RJ. 2012. A literature review on reaction time. http://biae.clemson.edu/bpc/bp/Lab/110/reaction.htm. Accessed 1 December 2012.
Kruschke JK. 2012. Complete example of right censoring in JAGS (with rjags). Doing Bayesian Data Analysis. http://doingbayesiandataanalysis.blogspot.com/2012/01/complete-example-of-right-censoring-in.html. Accessed: 1 December 2012.
Kuo Y-L (1989) Temporal analysis of oral, nasal, and retronasal perception of citral and vanillin. MS Thesis, Food Science and Technology, University of California, Davis CA
Kuo Y-L, Pangborn RM, Noble AC (1993) Temporal patterns of nasal, oral, and retronasal perception of citral and vanillin and interaction of these odourants with selected tastants. Internat J Food Sci Technol 28:137–137
Lagakos SW (1979) General right censoring and its impact on the analysis of survival data. Biometrics 35(1):139–156
Laing DG, MacLeod P (1992) Reaction time for the recognition of odor quality. Chem Senses 17:337–346
Leclercq S, Blancher G (2012) Multimodal sensory integration during sequential eating—linking chewing activity, aroma release, and aroma perception over time. Chem Senses 37:689–700
Lee K (1989a). Perception of irritation from ethanol, capsaicin, and cinnamyl aldehyde via nasal, oral, and retronasal pathways. MS Thesis, Food Sciences, University of California, Davis CA
Lee WE III (1989b) Single-point versus time-intensity sensory measurements: an informational entropy analysis. J Sensory Stud 4(1):19–30
Lee J, Halpern BP (2006) Retronasal and orthonasal adaptation: similar over 60 seconds. Chem Senses 31:479, abstract
Lee J, Halpern BP (2007) Time-intensity tracking of retronasal smelling. Chem Senses 32:A15, abstract
Lehrner JP, Glück J, Laska M (1999) Odor identification, consistency of label use, olfactory threshold and their relationships to odor memory over the human lifespan. Chem Senses 25:337–346
Miettinen S-M (2004) Instrumentally measured release and human perception of aroma compounds from foods and model systems differing in fat content. Academic Disseretation, Faculty of Agriculture and Forestry of the University of Helsinki
Miettinen S-M, Hyvönen L, Linforth RST, Taylor AJ, Tuorila H (2004) Temporal aroma delivery from milk systems containing 0–5 % added fat, observed by free choice profiling, time intensity, and atmospheric pressure chemical ionization–mass spectrometry techniques. J Ag Food Chem 52(26):8111–8118
Møller P, Köster EP, Dijkman N (2012) Same–different reaction times to odors: some unexpected findings. Chemo Percept 5:158–171
Moncrieff RW (1967) The chemical senses. CRC Press, Cleveland, p 615
O’Neil MJ Editor, Heckelman PE Senior Associate, Koch CB, Roman KJ Associate Editors. 2006. The Merck Index, 14 ed. Merck Research Laboratories, Rahway, NJ
Opet JM (1989) Effect of caffeine, ethanol, and sucrose on temporal perception of menthol. MS Thesis, University of California, Davis CA
Overbosch P, van den Enden JC, Keur BM (1986) An improved method for measuring perceived intensity/time relationships in human taste and smell. Chem Senses 11:331–338
Peters M, Ivanoff J (1999) Performance asymmetries in computer mouse control of right-handers, and left-handers with left-and right-handed mouse experience. J Motor Behav 31(1):86–94
Pew RW, Rosenbaum DA (1988) Human movement control. Computation, representation, and implementation. In: Atkinson RC, Herrnstein RJ, Lindzey G, Luce D (eds) Stevens’ handbook of experimental psychology, vol 2, 2nd edn, Learning and cognition. Wiley, New York, pp 473–509
Pierce J, Halpern BP (1996) Orthonasal and retronasal odorant identification based upon vapor phase input from common substances. Chem Senses 21:529–543
Pinching AJ (1977) Clinical testing of olfaction reassessed. Brain 100:377–388
Scott-Johnson PE, Blakley D, Scott JW (2000) Effects of air flow on rat electroolfactogram. Chem Senses 25:761–768
Shangari GK, Halpern BP (2005) Intensity of retronasal and orthonasal odorants: time–intensity tracking. Chem Senses 30:278, abstract
Shepherd GM (2011) Neurogastronomy. Columbia University Press, New York, p 288
Silver WL (1990) Physiological factors in nasal trigeminal chemoreception. In: Green BG, Mason JR, Kare MR (eds) Chemical senses, 2nd edn, Irritation. Decker, New York, pp 21–41
Small DM (2012) Flavor is in the brain. Physiol Behav 107(4):540–552
Small DM, Gerber JC, Mak YE, Hummel T (2005) Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron 47:593–605
Sood S, Halpern BP (2005) Time and intensity patterns of orthonasal and retronasal smelling. Chem Senses 30:A152, abstract
Soussignan R, Schaal B, Rigaud D, Royet J-P, Jiang T (2011) Hedonic reactivity to visual and olfactory cues: rapid facial electromyographic reactions are altered in anorexia nervosa. Biol Psychol 86(3):265–272
Stephenson D, Halpern BP (2009) No oral-cavity-only discrimination of purely olfactory odorants. Chem Senses 34:121–126
Stevens JC, Cain WS, Schiet FT, Oatley MW (1989) Olfactory adaptation and recovery in old age. Perception 18(2):265–276
Sun BC, Halpern BP (2005) Identification of air-phase retronasal and orthonasal odorant pairs. Chem Senses 30:1–14
Tucker D (1963) Physical variables in the olfactory stimulation process. J Gen Physiol 46:453–489
Voirol E, Daget N (1986) Comparative study of nasal and retronasal olfactory perception. Lebensm Wiss u Technol Food Sci Technol 19:316–319
Wilson DA (1997) Habituation of odor responses in the rat anterior piriform cortex. J Physiol 79:1425–1440
Wise PM, Wysocki CJ, Radil T (2003) Time–intensity ratings of nasal irritation from carbon dioxide. Chem Senses 28:751–760
Wright HN (1987) Characterization of olfactory dysfunction. Arch Otolaryngol Head Neck Surgery 113:163–168
Wu MC, Carroll RJ (1988) Estimation and comparison of changes in the presence of informative right censoring by modeling the censoring process. Biometrics 44(1):175–188
Zhong M, Hess KR (2009) Mean survival time from right censored data. COBRA Preprint Series. Working Paper 66. http://biostats.bepress.com/cobra/art66. Accessed 1 December 2012
Funding
This study was supported by the USDA Hatch NYC-191403, The Cornell Presidential Scholars Program, and a Susan Linn Sage Professorship.
Acknowledgments
We thank Rick Dale for creating the time–intensity-tracking computer program in REALbasic, Francine H. Hollis and Robert A. Raguso for comments on a previous draft of this manuscript, and the anonymous reviewers for their recommendations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, J., Halpern, B.P. High-Resolution Time–Intensity Tracking of Sustained Human Orthonasal and Retronasal Smelling During Natural Breathing. Chem. Percept. 6, 20–35 (2013). https://doi.org/10.1007/s12078-012-9136-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12078-012-9136-6