Abstract
Background
The prevalence of nonalcoholic fatty liver disease (NAFLD) and alcohol-associated/related liver disease (ALD) with metabolic syndrome is increasing globally. Metabolic syndrome and excessive alcohol consumption synergically exacerbate liver pathologies; therefore, drinking-specific serum markers unaffected by liver injury or metabolic syndrome are essential for assessing alcohol consumption. We evaluated the ratio of carbohydrate-deficient transferrin to total transferrin (%CDT) in patients with fatty liver disease, particularly focusing on its correlation with metabolic factors (UMIN000033550).
Methods
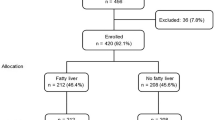
A total of 120 patients with fatty liver disease, including ALD and NAFLD, were screened for alcohol misuse using the Alcohol Use Disorders Identification Test. Associations of metabolic syndrome-related factors and hepatic steatosis/liver stiffness with drinking markers, such as %CDT, gamma-glutamyl transferase (GGT), and mean corpuscular volume (MCV), were assessed using multiple linear regression analyses.
Results
%CDT significantly increased with 3–4 drinks/day. The optimal cutoff value for identifying non- to light drinkers was 1.78% (sensitivity, 71.8%; specificity, 83.7%; and area under the receiver operating characteristic curve [AUROC], 0.851), which was significantly higher than that for GGT. The cutoff value for identifying heavy drinkers was 2.08% (sensitivity, 65.5%; specificity, 86.8%; and AUROC, 0.815). Multiple regression analysis revealed that this proportion was negatively correlated with body mass index, whereas GGT and MCV were influenced by multiple factors involved in liver injury and dyslipidemia.
Conclusions
%CDT showed a strong correlation with alcohol consumption, independent of liver damage, steatosis/stiffness, or metabolic syndrome-related factors, indicating that it is a useful drinking marker for the accurate diagnosis of NAFLD and ALD.
Graphical abstract


Similar content being viewed by others
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. The Lancet. 2019;393(10190):2493–2502
Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362:k2817
Tateishi R, Uchino K, Fujiwara N, Takehara T, Okanoue T, Seike M, et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J Gastroenterol. 2019;54(4):367–376
Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904
Suzuki M, Kon K, Ikejima K, Arai K, Uchiyama A, Aoyama T, et al. The chemical chaperone 4-phenylbutyric acid prevents alcohol-induced liver injury in obese KK-A(y) mice. Alcohol Clin Exp Res. 2019;43(4):617–627
Chang Y, Cho YK, Kim Y, Sung E, Ahn J, Jung H-S, et al. Nonheavy drinking and worsening of noninvasive fibrosis markers in nonalcoholic fatty liver disease: a cohort study. Hepatology. 2019;69(1):64–75
Stepanova M, Rafiq N, Younossi ZM. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 2010;59(10):1410–1415
Mahli A, Hellerbrand C. Alcohol and obesity: a dangerous association for fatty liver disease. Dig Dis. 2016;34(Suppl 1):32–39
Ikejima K, Kon K, Yamashina S. Nonalcoholic fatty liver disease and alcohol-related liver disease: from clinical aspects to pathophysiological insights. Clin Mol Hepatol. 2020;26(4):728–735
Bergstrom JP, Helander A. Clinical characteristics of carbohydrate-deficient transferrin (%disialotransferrin) measured by HPLC: sensitivity, specificity, gender effects, and relationship with other alcohol biomarkers. Alcohol Alcohol. 2008;43(4):436–441
Helander A, Husa A, Jeppsson JO. Improved HPLC method for carbohydrate-deficient transferrin in serum. Clin Chem. 2003;49(11):1881–1890
Tavakoli HR, Hull M, Michael OL. Review of current clinical biomarkers for the detection of alcohol dependence. Innov Clin Neurosci. 2011;8(3):26–33
Ohtsuka T, Tsutsumi M, Fukumura A, Tsuchishima M, Takase S. Use of serum carbohydrate-deficient transferrin values to exclude alcoholic hepatitis from non-alcoholic steatohepatitis: a pilot study. Alcohol Clin Exp Res. 2005;29(12 Suppl):236S-S239
Wang J, Li P, Jiang Z, Yang Q, Mi Y, Liu Y, et al. Diagnostic value of alcoholic liver disease (ALD)/nonalcoholic fatty liver disease (NAFLD) index combined with γ-glutamyl transferase in differentiating ALD and NAFLD. Korean J Intern Med. 2016;31(3):479–487
Sakutata H, Suzuki T, Yasuda H, Ito T. Beverage-specific effects of ethanol consumption on its biological markers. Clin Chem Lab Med. 2008;46(5):699–702
Delanghe JR, Helander A, Wielders JP, Pekelharing JM, Roth HJ, Schellenberg F, et al. Development and multicenter evaluation of the N latex CDT direct immunonephelometric assay for serum carbohydrate-deficient transferrin. Clin Chem. 2007;53(6):1115–1121
Szegedi A, Muller MJ, Himmerich H, Anghelescu I, Wetzel H. Carbohydrate-deficient transferrin (CDT) and HDL cholesterol (HDL) are highly correlated in male alcohol dependent patients. Alcohol Clin Exp Res. 2000;24(4):497–500
Hock B, Schwarz M, Domke I, Grunert VP, Wuertemberger M, Schiemann U, et al. Validity of carbohydrate-deficient transferrin (%CDT), gamma-glutamyltransferase (gamma-GT) and mean corpuscular erythrocyte volume (MCV) as biomarkers for chronic alcohol abuse: a study in patients with alcohol dependence and liver disorders of non-alcoholic and alcoholic origin. Addiction. 2005;100(10):1477–1486
Suzuki T, Eguchi A, Shigefuku R, Nagao S, Morikawa M, Sugimoto K, et al. Accuracy of carbohydrate-deficient transferrin as a biomarker of chronic alcohol abuse during treatment for alcoholism. Hepatol Res. 2021;2021:5
Hietala J, Koivisto H, Anttila P, Niemela O. Comparison of the combined marker GGT-CDT and the conventional laboratory markers of alcohol abuse in heavy drinkers, moderate drinkers and abstainers. Alcohol Alcohol. 2006;41(5):528–533
Liang SS, He Y, Huang ZG, Jia CY, Gan W. Evaluation of the diagnostic utility of carbohydrate-deficient transferrin in chronic alcoholism: Results from Southwest China. Med (Baltim). 2021;100(4):e24467
Fagan KJ, Irvine KM, McWhinney BC, Fletcher LM, Horsfall LU, Johnson L, et al. Diagnostic sensitivity of carbohydrate deficient transferrin in heavy drinkers. BMC Gastroenterol. 2014;14:97
Schellenberg F, Schwan R, Mennetrey L, Loiseaux MN, Pages JC, Reynaud M. Dose-effect relation between daily ethanol intake in the range 0–70 grams and %CDT value: validation of a cut-off value. Alcohol Alcohol. 2005;40(6):531–534
Lackner C, Spindelboeck W, Haybaeck J, Douschan P, Rainer F, Terracciano L, et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol. 2017;66(3):610–618
Whitfield JB, Dy V, Madden PA, Heath AC, Martin NG, Montgomery GW. Measuring carbohydrate-deficient transferrin by direct immunoassay: factors affecting diagnostic sensitivity for excessive alcohol intake. Clin Chem. 2008;54(7):1158–1165
Fagan KJ, Irvine KM, McWhinney BC, Fletcher LM, Horsfall LU, Johnson LA, et al. BMI but not stage or etiology of nonalcoholic liver disease affects the diagnostic utility of carbohydrate-deficient transferrin. Alcohol Clin Exp Res. 2013;37(10):1771–1778
Kalapatapu RK, Chambers R. Novel objective biomarkers of alcohol use: potential diagnostic and treatment management tools in dual diagnosis care. J Dual Diagn. 2009;5(1):57–82
Hultberg B, Isaksson A, Agardh E, Agardh CD. Plasma beta-hexosaminidase isoenzymes A and B exhibit different relations to blood glucose levels in a population of Type 1 diabetic patients. Scand J Clin Lab Invest. 1995;55(8):723–728
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209
Funding
None.
Author information
Authors and Affiliations
Contributions
Material preparation: MM, KK, AU, HF, KF, RY, EN, and SY; data collection and analysis: MM and KK; manuscript drafting: KK; experiment supervision and paper review: KI.
Corresponding author
Ethics declarations
Conflict of interest
Maki Morinaga, Kazuyoshi Kon, Akira Uchiyama, Hiroo Fukada, Kyoko Fukuhara, Reiko Yaginuma, Eisuke Nakadera, Shunhei Yamashina, and Kenichi Ikejima declare that they have no Conflict interests.
Informed consent in studies with human subjects
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Animal studies
This article does not contain any studies with animal subjects.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12072_2022_10298_MOESM1_ESM.tif
Supplementary Fig. 1 ROC curve of %CDT limited to women. a) The ROC curves for detecting non- or light drinkers. The area under the ROC curve was 0.843 (95% CI, 0.714−0.971) for %CDT. b) The ROC curves for detecting heavy drinkers. The area under the ROC curve was 0.839 (95% CI, 0.683−0.994) for %CDT (n=38). ROC, receiver operating characteristics; %CDT, carbohydrate-deficient transferrin to total transferrin; CI, confidence interval (TIF 76357 kb)
12072_2022_10298_MOESM2_ESM.tif
Supplementary Fig. 2 ROC curve of %CDT limited to patients with BMI ≥25 kg/m2 . a) The ROC curves for detecting non- or light drinkers. The area under the ROC curve was 0.884 (95% CI, 0.754−1.013) for %CDT. b) The ROC curves for detecting heavy drinkers. The area under the ROC curve was 0.938 (95% CI, 0.867−1.001) for %CDT (n=50). ROC, receiver operating characteristics; %CDT, carbohydrate-deficient transferrin to total transferrin; BMI, body mass index; CI, confidence interval (TIF 76357 kb)
12072_2022_10298_MOESM3_ESM.tif
Supplementary Fig. 3 ROC curve of %CDT limited to patients with cirrhosis. a) The ROC curves for detecting non- or light drinkers. The area under the ROC curve was 0.857 (95% CI, 0.619−1.095) for %CDT. b) The ROC curves for detecting heavy drinkers. The area under the ROC curve was 0.839 (95% CI, 0.652−1.027) for %CDT (n=18). ROC, receiver operating characteristics; %CDT, carbohydrate-deficient transferrin to total transferrin; CI, confidence interval (TIF 76359 kb)
Rights and permissions
About this article
Cite this article
Morinaga, M., Kon, K., Uchiyama, A. et al. Carbohydrate-deficient transferrin is a sensitive marker of alcohol consumption in fatty liver disease. Hepatol Int 16, 348–358 (2022). https://doi.org/10.1007/s12072-022-10298-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-022-10298-8