Abstract
Background/purpose
The study compared the hepatitis B surface antigen (HBsAg) changes between hepatitis B e antigen (HBeAg)-negative non-cirrhotic chronic hepatitis B patients who discontinued or maintained entecavir therapy.
Methods
A total of 250 HBeAg-negative, non-cirrhotic patients who were treated with entecavir previously and had stopped treatment for at least 12 months (discontinued group) and 231 HBeAg-negative, non-cirrhotic patients who had received entecavir treatment for at least 4 years (maintained group) were recruited.
Results
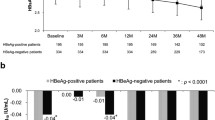
In the discontinued group, 71 had a persistent virological suppression (Group I), 35 experienced virological relapse but no clinical relapse or retreatment (Group II), 26 experienced clinical relapse without retreatment (Group III), and 118 experienced HBV relapse and retreatment (Group IV). Patients in Groups I, II, and III, but not in Group IV, experienced a significantly larger drop in HBsAg levels’ post-treatment than during entecavir treatment. Patients in Groups I and III exhibited a greater post-treatment HBsAg decline than patients in Groups II and IV (p < 0.001). Discontinued group experienced a significantly larger drop in HBsAg levels (p < 0.001) and higher HBsAg loss rate (p = 0.001) than maintained group after adjusting for clinical features and HBsAg levels in propensity score matched patients. Patients in maintained group exhibited a smaller drop in HBsAg and lower HBsAg loss rate than patients in groups I, II, and III, but not in Group IV.
Conclusions
Patients who discontinued entecavir therapy and achieved persistent virological suppression exhibited a greater HBsAg decline and higher HBsAg loss rate compared with patients who maintained entecavir therapy.


Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- CHB:
-
Chronic hepatitis B
- GEE:
-
Generalized estimating equations
- HBeAg:
-
Hepatitis B e antigen
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- NA:
-
Nucleos(t)ide analogue
- PS:
-
Propensity score
- ROC curve:
-
Receiver-operating characteristic curve
- SD:
-
Standard deviation
- ULN:
-
Upper limit of normal
References
Chen CH, Hu TH, Hung CH, Lu SN, Wang JH, Chang MH, et al. A comparison of four-year entecavir efficacy in nucleos(t)ide analogue-naïve and -experienced adult Taiwanese chronic hepatitis B patients. Hepatol Int. 2013;7:832–43.
Ono A, Suzuki F, Kawamura Y, Sezaki H, Hosaka T, Akuta N, et al. Long-term continuous entecavir therapy in nucleos(t)ide-naïve chronic hepatitis B patients. J Hepatol. 2012;57:508–14.
Jeng WJ, Sheen IS, Chen YC, Hsu CW, Chien RN, Chu CM, et al. Off therapy durability of response to entecavir therapy in hepatitis B e antigen negative chronic hepatitis B patients. Hepatology. 2013;58:1888–96.
Chen CH, Lu SN, Hung CH, Wang JH, Hu TH, Changchien CS, et al. The role of hepatitis B surface antigen quantification in predicting HBsAg loss and HBV relapse after discontinuation of lamivudine treatment. J Hepatol. 2014;61:515–22.
Chen CH, Hung CH, Hu TH, Wang JH, Lu SN, Su PF, et al. Association between level of hepatitis B surface antigen and relapse after entecavir therapy for chronic HBV infection. Clin Gastroenterol Hepatol. 2015;13:1984–92.
Chen CH, Hung CH, Wang JH, Lu SN, Hu TH, Lee CM. Long-term incidence and predictors of hepatitis B surface antigen loss after discontinuing nucleoside analogues in noncirrhotic chronic hepatitis B patients. Clin Microbiol Infect. 2018;24:997–1003.
Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology. 2012;143:629–36.
Hung CH, Wang JH, Lu SN, Hu TH, Lee CM, Chen CH. Hepatitis B surface antigen loss and clinical outcomes between HBeAg-negative cirrhosis patients who discontinued or continued nucleoside analogue therapy. J Viral Hepat. 2017;24:599–607.
Chen CH, Hung CC, Wang JH, Lu SN, Lai HC, Hu TH, et al. The incidence of hepatitis B surface antigen loss between hepatitis B e antigen-negative non-cirrhotic patients who discontinued or continued entecavir therapy. J Infect Dis. 2019;219:1624–33.
Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–61.
Hung CH, Lu SN, Wang JH, Lee CM, Chen TM, Tung HD, et al. Correlation between ultrasonographic and pathologic diagnoses of hepatitis B and C virus-related cirrhosis. J Gastroenterol. 2003;38:153–7.
Lee CM, Chen CH, Lu SN, Tung HD, Chou WJ, Wang JH, et al. Prevalence and clinical implications of hepatitis B virus genotypes in southern Taiwan. Scand J Gastroenterol. 2003;38:95–101.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98.
Berg T, Simon KG, Mauss S, Schott E, Heyne R, Klass DM, et al. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients—FINITE study. J Hepatol. 2017;67:918–24.
Boni C, Laccabue D, Lampertico P, Giuberti T, Viganò M, Schivazappa S, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963–73.
Rinker F, Zimmer C, Höner Zu Siederdissen C, Manns MP, Kraft ARM, Wedemeyer H, et al. Hepatitis B virus-specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Hepatol. 2018;69:584–93.
Zimmer CL, Rinker F, Höner Zu Siederdissen C, Manns MP, Wedemeyer H, et al. Increased NK cell function after cessation of long-term nucleos(t)ide analogue treatment in chronic hepatitis B is associated with liver damage and HBsAg loss. J Infect Dis. 2018;217:1656–66.
Liu J, Li T, Zhang L, Xu A. The role of hepatitis B surface antigen in nucleos(t)ide analogues cessation among Asian chronic hepatitis B patients: a systematic review. Hepatology. 2019;70(3):1045–55.
Su TH, Hu TH, Chen CY, Huang YH, Chuang WL, Lin CC, et al. Four- year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016;36:1755–64.
Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107.
Funding
This study was supported by Grants CMRPG8G1281 from the Chang Gung Memorial Hospital, Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Cheng-Yuan Peng has served as an advisory committee member for AbbVie, BMS, Gilead, MSD, and Roche. The coauthors Chien-Hung Chen, Tsung-Hui Hu, Jing-Houng Wang, Hsueh-Chou Lai, Chao-Hung Hung, Sheng-Nan Lu have no conflicts of interest to declare.
Ethical approval
This study was conducted in accordance with the 1975 Declaration of Helsinki as revised in 2008. Informed consent was obtained from all patients. The study was approved by the Research Ethics Committees of Chang Gung Memorial Hospital and China Medical University Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig 1.
Flow chart of study population (TIFF 1482 kb)
Supplementary Fig 2.
Comparison of HBsAg changes during and post-entecavir treatment between Group I patients (persistent viral suppression) and Groups II and III patients who achieved persistent viral suppression after transient virological or clinical relapse (TIFF 29 kb)
Rights and permissions
About this article
Cite this article
Chen, CH., Hu, TH., Wang, JH. et al. Comparison of HBsAg changes between HBeAg-negative patients who discontinued or maintained entecavir therapy. Hepatol Int 14, 317–325 (2020). https://doi.org/10.1007/s12072-019-09991-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-09991-y