Abstract
Background
Hepatitis C virus (HCV) requires epidemiological monitoring to estimate its disease burden and to develop countermeasures. This study aimed to investigate the difference between the 2015 and 2009 nationwide anti-HCV seroprevalence and to determine linkage to care estimates in South Korea.
Methods
A total 268,422 examinees ≥ 20 years old were included in 2015 from 33 medical institutions nationwide. Electronically extracted data were retrospectively analyzed to calculate the age-, sex-, and area-adjusted anti-HCV prevalence. Seroprevalence in 2015 was measured using the same method as that in 2009. For anti-HCV-positive subjects, medical records were reviewed to see whether HCV RNA testing or antiviral treatment was performed.
Results
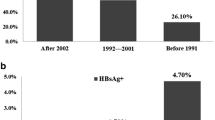
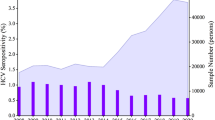
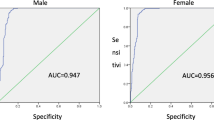
Adjusted anti-HCV prevalence was 0.60% (95% confidence interval, 0.57–0.63) based on general Korean population in 2015. It showed an increasing trend according to age; 0.23% in thirties, 0.38% in forties, 0.63% in fifties, 1.08% in sixties, and 1.65% in those aged ≥ 70 years. From 2009 to 2015, the adjusted anti-HCV prevalence decreased by 30%, with odds ratio of 0.70 (95% CI 0.70–0.71). There was significant intranational regional variation and changing pattern of seroprevalence. Among 1359 anti-HCV-positive subjects, HCV RNA test was performed in 60% and 25.4% had positivity. Treatment-initiated and cured rates in 2015 were 18.5% and 10.9%, respectively.
Conclusions
Anti-HCV prevalence in South Korea was 0.6% in 2015, showing a 30% decrease from that in 2009. Although the HCV RNA testing rate was increased since 2009, this remains suboptimal. Moreover, the treatment uptake rate should be improved in South Korea.



Similar content being viewed by others
Abbreviations
- HCV:
-
Hepatitis C virus
- RNA:
-
Ribonucleic acid
- DAA:
-
Direct acting antiviral drug
- SVR:
-
Sustained virologic response
- AST:
-
Aspartate aminotransferases
- ALT:
-
Alanine aminotransferases
- GGT:
-
Gamma-glutamyl transferase
- CI:
-
Confidence interval
- HLA:
-
Human leukocyte antigen
- IL:
-
Interleukin
References
Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–62.
Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–57.
Hajarizadeh B, Grebely J, Martinello M, Matthews GV, Lloyd AR, Dore GJ. Hepatitis C treatment as prevention: evidence, feasibility, and challenges. Lancet Gastroenterol Hepatol. 2016;1:317–27.
World Health Organization. Global Hepatitis Report 2017, 2017.
World Health Organization. Combating hepatitis B and C to reach elimination by 2030, 2016.
Kim DY, Kim IH, Jeong SH, Cho YK, Lee JH, Jin YJ, Lee D, Suh DJ, Han KH, Park NH, Kang HY, Jung YK, Kim YS, Kim KA, Lee YJ, Lee BS, Yim HJ, Lee HJ, Baik SK, Tak WY, Lee SJ, Chung WJ, Choi SK, Cho EY, Heo J, Kim DJ, Song BC, Kim MW, Lee J, Chae HB, Choi DH, Choi HY, Ki M. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int. 2013;33:586–94.
Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–61.
Yehia BR, Schranz AJ, Umscheid CA, Lo Re V III. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9:e101554.
Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–62.
Kwon GY, Lee H, Gwack J, Lee SW, Ki M, Youn SK. Regional distribution of hepatitis C virus infection in the Republic of Korea, 2007-2011. Gut Liver. 2014;8:428–32.
Jeong SH, Jang ES, Choi HY, Kim KA, Chung W, Ki M. Current status of hepatitis C virus infection and countermeasures in South Korea. Epidemiol Health. 2017;39:e2017017.
Sohn HS, Kim JR, Ryu SY, Lee YJ, Lee MJ, Min HJ, Lee J, Choi HY, Song YJ, Ki M. Risk factors for hepatitis C virus (HCV) infection in areas with a high prevalence of HCV in the Republic of Korea in 2013. Gut Liver. 2016;10:126–32.
Kim JY, Cho J, Hwang SH, Kil H, Bae SH, Kim YS, Lee HC, Jeong SH. Behavioral and healthcare-associated risk factors for chronic hepatitis C virus infection in Korea. J Korean Med Sci. 2012;27:1371–7.
Seong MH, Kil H, Kim YS, Bae SH, Lee YJ, Lee HC, Kang BH, Jeong SH. Clinical and epidemiological features of hepatitis C virus infection in South Korea: a prospective, multicenter cohort study. J Med Virol. 2013;85:1724–33.
Shin A, Cho ER, Kim J, Sung J, Park KW, Lim MK, Shin HR. Factors associated with awareness of infection status among chronic hepatitis B and C carriers in Korea. Cancer Epidemiol Biomark Prev. 2009;18:1894–8.
Zuckerman A, Douglas A, Nwosu S, Choi L, Chastain C. Increasing success and evolving barriers in the hepatitis C cascade of care during the direct acting antiviral era. PLoS One. 2018;13:e0199174.
Chirikov VV, Marx SE, Manthena SR, Strezewski JP, Saab S. Development of a comprehensive dataset of hepatitis C patients and examination of disease epidemiology in the United States, 2013–2016. Adv Ther. 2018;35:1087–102.
Viejo LG, Herola AG, Lloret IS, Ruano FS, Paulino IC, Ivorra CQ, Saavedra IA, Perez DM, de la Osa JV. Screening of hepatitis C virus infection in adult general population in Spain. Eur J Gastroenterol Hepatol. 2018;30:1077–81.
Huang P, Dong L, Lu X, Zhang Y, Chen H, Wang J, Zhang Y, Su J, Yu R. Genetic variants in antigen presentation-related genes influence susceptibility to hepatitis C virus and viral clearance: a case control study. BMC Infect Dis. 2014;14:716.
Jeong SH, Jung YK, Yang JW, Park SJ, Kim JW, Kwon OS, Kim YS, Choi DJ, Kim JH. Efficacy of peginterferon and ribavirin is associated with the IL28B gene in Korean patients with chronic hepatitis C. Clin Mol Hepatol. 2012;18:360–7.
Jimenez-Sousa MA, Fernandez-Rodriguez A, Guzman-Fulgencio M, Garcia-Alvarez M, Resino S. Meta-analysis: implications of interleukin-28B polymorphisms in spontaneous and treatment-related clearance for patients with hepatitis C. BMC Med. 2013;11:6.
Huang J, Huang K, Xu R, Wang M, Liao Q, Xiong H, Li C, Tang X, Shan Z, Zhang M, Rong X, Nelson K, Fu Y. The associations of HLA-A*02:01 and DRB1*11:01 with hepatitis C virus spontaneous clearance are independent of IL28B in the Chinese population. Sci Rep. 2016;6:31485.
Lee SS, Jeong SH, Jang ES, Kim YS, Lee YJ, Jung EU, Kim IH, Bae SH, Lee HC. Treatment rate and factors related to interferon-based treatment initiation for chronic hepatitis C in South Korea. J Med Virol. 2016;88:275–81.
Jang ES, Kim YS, Kim KA, Lee YJ, Chung WJ, Kim IH, Lee BS, Jeong SH. Final report of unmet needs of interferon-based therapy for chronic hepatitis C in Korea: basis for moving into the direct-acting antiviral era. Gut Liver. 2017;11:543–50.
Janjua NZ, Kuo M, Yu A, Alvarez M, Wong S, Cook D, Wong J, Grebely J, Butt ZA, Samji H, Ramji A, Tyndall M, Krajden M. The population level cascade of care for hepatitis C in British Columbia, Canada: the BC hepatitis testers cohort (BC-HTC). EBioMedicine. 2016;12:189–95.
Simmons R, Ireland G, Irving W, Hickman M, Sabin C, Ijaz S, Ramsay M, Lattimore S, Mandal S. Establishing the cascade of care for hepatitis C in England-benchmarking to monitor impact of direct acting antivirals. J Viral Hepat. 2018;25:482–90.
Acknowledgements
This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (Grant number: HC15C1193) and by a grant of the Chronic Infectious Disease Cohort Study (Korea HCV Cohort Study, 4800-4859-304) from the Korea Centers for Disease Control, Republic of Korea. The Korean hepatitis epidemiology study group included: Eun Sun Jang: Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam, Gyeonggi, Moran Ki: Department of Cancer Control and Population Health, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Gyeonggi, Geum-Youn Gwak: Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Kyung-Ah Kim: Department of Internal Medicine, Inje University Ilsan Paik Hospital, Goyang, Gyeonggi, Gi-Ae Kim: Health Screening and Promotion Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul. Do Young Kim: Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Dong Joon Kim: Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Gangwon, Man Woo Kim: Department of Internal Medicine, Chosun University School of Medicine, Gwangju, Sung Eun Kim: Department of Internal Medicine, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Gyeonggi, Yun Soo Kim: Department of Internal Medicine, Gachon College of Medicine, Incheon, Young Seok Kim: Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Gyeonggi, In Hee Kim: Department of Internal Medicine, Chonbuk National University Hopital, Chonbuk National University College of Medicine, Chonju, Republic of Korea, Chang Wook Kim: Department of Internal Medicine, Uijeongbu St.Mary’s Hospital, Catholic University College of Medicine, Uijeongbu, Gyeonggi, Ho Dong Kim: Department of Internal Medicine, St.Carollo General Hospital, Suncheon, Jeonnam, Hyung Joon Kim: Department of Internal Medicine, Chung-Ang University Hospital, Chungang University College of Medicine, Seoul, Neung Hwa Park: Department of Internal Medicine, University of Ulsan College of Medicine, Ulsan, Soon Koo Baik: Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Gangwon, Jeong Ill Suh: Department of Internal Medicine, Dongguk University Gyeongju Hospital, Dongguk University College of Medicine, Gyeongju, Gyeongbuk, Byung-Cheol Song: Department of Internal Medicine, Jeju National University College of Medicine, Jeju, Il Han Song: Department of Internal Medicine, Dankook University Hospital, Cheonan, Chungnam, Jong Eun Yeon: Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Byung Seok Lee: Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Republic of Korea, Youn Jae Lee: Department of Internal Medicine, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Republic of Korea, Young Kul Jung: Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Gyeonggi, Woo Jin Chung: Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Sung Bum Cho: Department of Internal Medicine, Chonnam National University Hwasun Hospital, Jeonnam, Eun-Young Cho: Department of Internal Medicine, Wonkwang University College of Medicine, Iksan, Jeonbuk, Hyun Chin Cho: Department of Internal Medicine, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju, Gyeongnam, Gab Jin Cheon: Department of Internal Medicine, GangNeung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Gangwon, Hee Bok Chae: Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Chungbuk, Dae Hee Choi: Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Gangwon, Sung-Kyu Choi: Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Hwa Young Choi: Department of Cancer Control and Population Health, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Gyeonggi, Won Young Tak: Department of Internal Medicine, Kyungpook National University College of Medicine, Daegu, Jeong Heo: Department of Internal Medicine, Pusan National University School of Medicine, Busan, Sook-Hyang Jeong: Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam, Gyeonggi.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Eun Sun Jang, Moran Ki, Hwa Young Choi, Kyung-Ah Kim and Sook-Hyang Jeong have nothing to declare.
Ethical approval
The study protocol was reviewed and approved by the institutional review board of each hospital.
Informed consent
Requirement for informed consent was waived due to the retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of the Korean hepatitis epidemiology study group are listed in the Acknowledgements section.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jang, E.S., Ki, M., Choi, H.Y. et al. The change in the nationwide seroprevalence of hepatitis C virus and the status of linkage to care in South Korea from 2009 to 2015. Hepatol Int 13, 599–608 (2019). https://doi.org/10.1007/s12072-019-09975-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-09975-y