Abstract
Background
Switching from nucleos(t)ide analogues to interferon (IFN) improves hepatitis B surface antigen (HBsAg) loss. We aimed to evaluate whether combining immunomodulators such as interleukin-2 (IL-2) and therapeutic vaccine with IFN enhances HBsAg loss in entecavir (ETV)-suppressed patients.
Methods
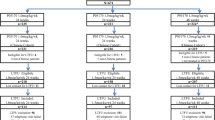
Ninety-four patients exhibiting virological suppression and hepatitis B e antigen (HBeAg) loss following ETV treatment were randomized 1:1:1 to receive ETV (group I) or IFN (group II) for 48 weeks, or IFN and vaccine for 48 weeks plus IL-2 for 12 weeks (group III). The primary endpoint was HBsAg loss at week 48. Peripheral natural killer (NK) cells and regulatory T cells (Treg) were measured as immune checkpoint indicators.
Results
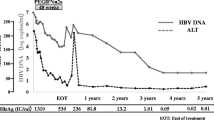
Mean HBsAg decline at week 48 was significantly greater in group III (0.85 log 10 IU/mL) and group II (0.74 log 10 IU/mL), than in group I (0.13 log 10 IU/mL). At week 48, 9.38%, 3.03%, and 3.70% of subjects in group III, II, and I, respectively, achieved HBsAg loss. Among patients with baseline HBsAg titers ranging from 100 to 1500 IU/mL, HBsAg loss rate was 27.3, 7.1, and 0% in group III, II, and I, respectively. Responders in group III showed a significantly higher increase in CD56bright CD16−NK cells from week 24 to 36, and a significant decline in Treg from week 12 to 24 than non-responders.
Conclusion
For ETV-suppressed patients, particularly those with low baseline HBsAg levels, combination therapy with IFN and other immunomodulators may enhance HBsAg loss, while successful response correlates with partial restoration of NK cells and Tregs.





Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- CHB:
-
Chronic hepatitis B
- cccDNA:
-
Covalently closed circular DNA
- ETV:
-
Entecavir
- HBeAg:
-
Hepatitis B e antigen
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- ITT:
-
Intention-to-treat
- IFN:
-
Interferon
- IL-2:
-
Interleukin-2
- LAM:
-
Lamivudine
- mITT:
-
Modified intention-to-treat
- NK cell:
-
Natural killer cell
- NA:
-
Nucleos(t)ide analogue
- Peg-IFN:
-
Pegylated Interferon
- ROC:
-
Receiver-operating characteristic
- Treg:
-
Regulatory T cell
- ULN:
-
Upper limit of normal
References
Dandri M, Locarnini S. New insight in the pathobiology of hepatitis B virus infection. Gut 2012;61(Suppl 1):i6–17
Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAg seroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology 2002;123:1084–1089
Buster EH, Flink HJ, Cakaloglu Y, Simon K, Trojan J, Tabak F, et al. Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology 2008;135:459–467
Moucari R, Korevaar A, Lada O, Martinot-Peignoux M, Boyer N, Mackiewicz V, et al. High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B patients responding to interferon: a long-term follow-up study. J Hepatol 2009;50:1084–1092
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL. Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;2017(67):370–398
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98
Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH, American Association for the Study of Liver D. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–283
Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005;352:2682–2695
Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004;351:1206–1217
Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 2010;51:422–430
Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008;359:2442–2455
Lin CL, Kao JH. Review article: novel therapies for hepatitis B virus cure—advances and perspectives. Aliment Pharmacol Ther 2016;44:213–222
Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, et al. Combination of tenofovir disoproxil fumarate and peginterferon alpha-2a increases loss of hepatitis b surface antigen in patients with chronic hepatitis B. Gastroenterology 2016;150(134–44):e10
Ning Q, Han M, Sun Y, Jiang J, Tan D, Hou J, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol 2014;61:777–784
Hu P, Shang J, Zhang W, Gong G, Li Y, Chen X, et al. HBsAg loss with peg-interferon alfa-2a in hepatitis b patients with partial response to nucleos(t)ide analog: new switch study. J Clin Transl Hepatol 2018;6:25–34
Ouzan D, Penaranda G, Joly H, Khiri H, Pironti A, Halfon P. Add-on peg-interferon leads to loss of HBsAg in patients with HBeAg-negative chronic hepatitis and HBV DNA fully suppressed by long-term nucleotide analogs. J Clin Virol 2013;58:713–717
Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology 2008;134:1938–1949
Boni C, Laccabue D, Lampertico P, Giuberti T, Vigano M, Schivazappa S, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology 2012;143(963–73):e9
Micco L, Peppa D, Loggi E, Schurich A, Jefferson L, Cursaro C, et al. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-alpha therapy of chronic hepatitis B. J Hepatol 2013;58:225–233
de Niet A, Stelma F, Jansen L, Sinnige MJ, Remmerswaal EB, Takkenberg RB, et al. Restoration of T cell function in chronic hepatitis B patients upon treatment with interferon based combination therapy. J Hepatol 2016;64:539–546
Yan W, Wu D, Wang X, Chen T, Lai Q, Zheng Q, et al. Upregulation of NKG2C + natural killer cells, TLR-2 expression on monocytes and downregulation of regulatory T-cells influence PEG-IFN treatment efficacy in entecavir-suppressed patients with CHB. Antivir Ther 2015;20:591–602
Qiu K, Liu B, Li SY, Li H, Chen ZW, Luo AR, et al. Systematic review with meta-analysis: combination treatment of regimens based on pegylated interferon for chronic hepatitis B focusing on hepatitis B surface antigen clearance. Aliment Pharmacol Ther 2018;47:1340–1348
Wu D, Ning Q. Toward a Cure for Hepatitis B Virus Infection: combination Therapy Involving Viral Suppression and Immune Modulation and Long-term Outcome. J Infect Dis 2017;216:S771–S777
Thimme R, Dandri M. Dissecting the divergent effects of interferon-alpha on immune cells: time to rethink combination therapy in chronic hepatitis B? J Hepatol 2013;58:205–209
Wu D, Han M, Ning Q. An integration of deep viral suppression with sequential immune modulation (cocktail therapy) to restore antiviral capacity: the future of chronic hepatitis B? J Hepatol 2015;62:240–241
Horiike N, Fazle Akbar SM, Michitaka K, Joukou K, Yamamoto K, Kojima N, et al. In vivo immunization by vaccine therapy following virus suppression by lamivudine: a novel approach for treating patients with chronic hepatitis B. J Clin Virol 2005;32:156–161
Kakumu S, Fuji A, Yoshioka K, Tahara H, Ohtani Y, Hirofuji H, et al. Pilot study of recombinant human interleukin 2 for chronic type B hepatitis. Hepatology 1988;8:487–492
Couillin I, Pol S, Mancini M, Driss F, Brechot C, Tiollais P, et al. Specific vaccine therapy in chronic hepatitis B: induction of T cell proliferative responses specific for envelope antigens. J Infect Dis 1999;180:15–26
Dahmen A, Herzog-Hauff S, Bocher WO, Galle PR, Lohr HF. Clinical and immunological efficacy of intradermal vaccine plus lamivudine with or without interleukin-2 in patients with chronic hepatitis B. J Med Virol 2002;66:452–460
Wursthorn K, Lutgehetmann M, Dandri M, Volz T, Buggisch P, Zollner B, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology 2006;44:675–684
Thompson AJ, Nguyen T, Iser D, Ayres A, Jackson K, Littlejohn M, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology 2010;51:1933–1944
Sonneveld MJ, Rijckborst V, Boucher CA, Hansen BE, Janssen HL. Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology 2010;52:1251–1257
Lampertico P, Vigano M, Di Costanzo GG, Sagnelli E, Fasano M, Di Marco V, et al. Randomised study comparing 48 and 96 weeks peginterferon alpha-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut 2013;62:290–298
Huang J, Zhang K, Chen W, Liao J, Luo X, Chen R. Switching to PegIFNalpha-2b leads to HBsAg loss in patients with low HBsAg levels and HBV DNA suppressed by NAs. Sci Rep 2017;7:13383
Honer Zu Siederdissen C, Cornberg M. The role of HBsAg levels in the current management of chronic HBV infection. Ann Gastroenterol 2014;27:105–112
Chevaliez S, Hezode C, Bahrami S, Grare M, Pawlotsky JM. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol 2013;58:676–683
Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006;354:1001–1010
Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, et al. IFN-alpha inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest 2012;122:529–537
Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 2014;343:1221–1228
Han M, Jiang J, Hou J, Tan D, Sun Y, Zhao M, et al. Sustained immune control in HBeAg-positive patients who switched from entecavir therapy to pegylated interferon-alpha2a: 1 year follow-up of the OSST study. Antivir Ther 2016;21:337–344
Boglione L, D’Avolio A, Cariti G, Milia MG, Simiele M, De Nicolo A, et al. Sequential therapy with entecavir and PEG-INF in patients affected by chronic hepatitis B and high levels of HBV-DNA with non-D genotypes. J Viral Hepat 2013;20:e11–e19
Moucari R, Boyer N, Ripault MP, Castelnau C, Mackiewicz V, Dauvergne A, et al. Sequential therapy with adefovir dipivoxil and pegylated interferon alfa-2a for HBeAg-negative patients. J Viral Hepat 2011;18:580–856
Nishioka M, Kagawa H, Shirai M, Terada S, Watanabe S. Effects of human recombinant interleukin 2 in patients with chronic hepatitis B: a preliminary report. Am J Gastroenterol 1987;82:438–442
Mizoguchi Y, Shin T, Sakagami Y, Seki S, Kuroki T, Kobayashi K, et al. Effects of recombinant interleukin 2 on immunological effector cells of the peripheral blood in patients with HBe antigen-positive chronic hepatitis. Gastroenterol Jpn 1988;23:147–152
Artillo S, Pastore G, Alberti A, Milella M, Santantonio T, Fattovich G, et al. Double-blind, randomized controlled trial of interleukin-2 treatment of chronic hepatitis B. J Med Virol 1998;54:167–172
Bruch HR, Korn A, Klein H, Markus R, Malmus K, Baumgarten R, et al. Treatment of chronic hepatitis B with interferon alpha-2b and interleukin-2. J Hepatol 1993;17(Suppl 3):S52–S55
Lunemann A, Lunemann JD, Munz C. Regulatory NK-cell functions in inflammation and autoimmunity. Mol Med 2009;15:352–358
Shi A, Zhang X, Xiao F, Zhu L, Yan W, Han M, et al. CD56(bright) natural killer cells induce HBsAg reduction via cytolysis and cccDNA decay in long-term entecavir-treated patients switching to peginterferon alfa-2a. J Viral Hepat 2018;25:1352–1362
Gill US, Peppa D, Micco L, Singh HD, Carey I, Foster GR, et al. Interferon alpha induces sustained changes in NK cell responsiveness to hepatitis B viral load suppression in vivo. PLoS Pathog 2016;12:e1005788
Kosinska AD, Pishraft-Sabet L, Wu W, Fang Z, Lenart M, Chen J, et al. Low hepatitis B virus-specific T-cell response in males correlates with high regulatory T-cell numbers in murine models. Hepatology 2017;66:69–83
Tilg H, Vogel W, Tratkiewicz J, Aulitzky WE, Herold M, Gruber M, et al. Pilot study of natural human interleukin-2 in patients with chronic hepatitis B immunomodulatory and antiviral effects. J Hepatol 1993;19:259–267
Acknowledgements
The authors would like to thank all the patients in this study, and the nurses who assisted in the patient management and the collection of serum samples. Data management and clinical trial monitoring were provided by Hangzhou Tigermed Consulting Co., Ltd.
Funding
This work was supported by grants from the Chinese National Twelfth Five Years Project in Science and Technology (2013ZX10002003), the Chinese National Thirteenth Five Years Project in Science and Technology (2017ZX10202201) and Hubei Provincial Natural Science Foundation of China (2018CFB206).
Author information
Authors and Affiliations
Contributions
QN, DW, and MH developed the concepts and designed the experiments. DW, and PW analyzed and interpreted the data. DW drafted the manuscript. YC, XC, QX, WY, CZ, QX, JJ, LW, DT, XD, YY, and JH were involved in patient recruitment, and data collection. QN, DW, XL, MH, and XW revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was performed in accordance with Good Clinical Practice and the Declaration of Helsinki principles for ethical research. The study protocol was approved by the independent central ethics committee of Tongji Medical College at Wuhan. Written informed consent was obtained from each participant. ClinicalTrials.gov Identifier: NCT02360592. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, D., Wang, P., Han, M. et al. Sequential combination therapy with interferon, interleukin-2 and therapeutic vaccine in entecavir-suppressed chronic hepatitis B patients: the Endeavor study. Hepatol Int 13, 573–586 (2019). https://doi.org/10.1007/s12072-019-09956-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-09956-1