Abstract
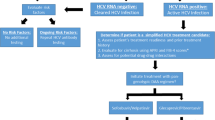
With the arrival of all-oral directly acting antiviral (DAA) therapy with high cure rates, the promise of hepatitis C virus (HCV) eradication is within closer reach. The availability of generic DAAs has improved access to countries with constrained resources. However, therapy is only one component of the HCV care continuum, which is the framework for HCV management from identifying patients to cure. The large number of undiagnosed HCV cases is the biggest concern, and strategies to address this are needed, as risk factor screening is suboptimal, detecting <20% of known cases. Improvements in HCV confirmation through either reflex HCV RNA screening or ideally a sensitive point of care test are needed. HCV notification (e.g., Australia) may improve diagnosis (proportion of HCV diagnosed is 75%) and may lead to benefits by increasing linkage to care, therapy and cure. Evaluations for cirrhosis using non-invasive markers are best done with a biological panel, but they are only moderately accurate. In resource-constrained settings, only generic HCV medications are available, and a combination of sofosbuvir, ribavirin, ledipasvir or daclatasvir provides sufficient efficacy for all genotypes, but this is likely to be replaced with pangenetypic regimens such as sofosbuvir/velpatasvir and glecaprevir/pibrentaasvir. In conclusion, HCV management in resource-constrained settings is challenging on multiple fronts because of the lack of infrastructure, facilities, trained manpower and equipment. However, it is still possible to make a significant impact towards HCV eradication through a concerted effort by individuals and national organisations with domain expertise in this area.




Similar content being viewed by others
References
Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333–42. doi:10.1002/hep.26141
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi:10.1016/S0140-6736(12)61728-0
Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61(1 Suppl):S45–57. doi:10.1016/j.jhep.2014.07.027
Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31(Suppl 2):61–80. doi:10.1111/j.1478-3231.2011.02540.x
Updated Income Classifications (Internet). World Bank; 2014. http://data.worldbank.org/news/2015-country-classifications. Cited 26 April 16
Medicines Patent Pool. About the Medicines Patent Pool. http://www.medicinespatentpool.org/about/. Accessed 26 Dec 2016
Lim SG. Chronic hepatitis C genotype 1 treatment roadmap for resource constrained settings. World J Gastroenterol. 2015;21(6):1972–81. doi:10.3748/wjg.v21.i6.1972
Organisation WH, editor. East African inter-country meeting on strengthening capacity for post-market surveillance of diagnostics; Nairobi: World Health Organisation; 2010. http://www.who.int/diagnostics_laboratory/quality/101115_report_east_african_meeting.pdf?ua=1
Organisation WH. Hepatitis C test kit evaluations. Geneva: World Health Organisation. http://www.who.int/diagnostics_laboratory/evaluations/hepc/en/. 7 July 2016
Organisation WH. Selecting and purchasing HIV, HBsAg and HCV in vitro diagnostics. World Health Organisation. http://www.who.int/entity/diagnostics_laboratory/procurement/purchase/en/index.html. 7 July 2016
World Health Organisation. Guidelines for the screening care and treatment of persons with chronic hepatitis C infection: updated version. Geneva; 2016
Al Olaby RR, Azzazy HM. Hepatitis C virus RNA assays: current and emerging technologies and their clinical applications. Expert Rev Mol Diagn. 2011;11(1):53–64. doi:10.1586/erm.10.101
Pisani G, Cristiano K, Marino F, Luciani F, Bisso GM, Mele C, et al. Quantification of hepatitis C virus (HCV) RNA in a multicenter study: implications for management of HCV genotype 1-infected patients. J Clin Microbiol. 2009;47(9):2931–6. doi:10.1128/JCM.00532-09
Chapko MK, Dufour DR, Hatia RI, Drobeniuc J, Ward JW, Teo CG. Cost-effectiveness of strategies for testing current hepatitis C virus infection. Hepatology. 2015;62(5):1396–404. doi:10.1002/hep.27966
Johannessen A. Where we are with point-of-care testing. J Viral Hepat. 2015;22(4):362–5. doi:10.1111/jvh.12385
Freiman JM, Tran TM, Schumacher SG, White LF, Ongarello S, Cohn J, et al. Hepatitis C core antigen testing for diagnosis of hepatitis C virus infection: a systematic review and meta-analysis. Ann Intern Med. 2016. doi:10.7326/M16-0065
Viner K, Kuncio D, Newbern EC, Johnson CC. The continuum of hepatitis C testing and care. Hepatology. 2015;61(3):783–9. doi:10.1002/hep.27584
Yehia BR, Schranz AJ, Umscheid CA, Lo Re V 3rd. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9(7):e101554. doi:10.1371/journal.pone.0101554
Hajarizadeh B, Grebely J, McManus H, Estes C, Razavi H, Gray RT, et al. Chronic hepatitis C burden and care cascade in Australia in the era of interferon-based treatment. J Gastroenterol Hepatol. 2016. doi:10.1111/jgh.13453
Gidding HF, Topp L, Middleton M, Robinson K, Hellard M, McCaughan G, et al. The epidemiology of hepatitis C in Australia: notifications, treatment uptake and liver transplantations, 1997–2006. J Gastroenterol Hepatol. 2009;24(10):1648–54
Eckman MH, Talal AH, Gordon SC, Schiff E, Sherman KE. Cost-effectiveness of screening for chronic hepatitis C infection in the United States. Clin Infect Dis. 2013;56(10):1382–93. doi:10.1093/cid/cit069
Smith BD, Yartel AK, Krauskopf K, Massoud OI, Brown KA, Fallon MB, et al. Hepatitis C virus antibody positivity and predictors among previously undiagnosed adult primary care outpatients: cross-sectional analysis of a multisite retrospective cohort study. Clin Infect Dis. 2015;60(8):1145–52. doi:10.1093/cid/civ002
Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(RR4):1–32
Southern WN, Norton B, Steinman M, DeLuca J, Drainoni ML, Smith BD, et al. A Birth-cohort testing intervention identified hepatitis c virus infection among patients with few identified risks: a cross-sectional study. BMC Infect Dis. 2015;15:553. doi:10.1186/s12879-015-1283-3
Meyer JP, Moghimi Y, Marcus R, Lim JK, Litwin AH, Altice FL. Evidence-based interventions to enhance assessment, treatment, and adherence in the chronic hepatitis C care continuum. Int J Drug Policy. 2015;26(10):922–35. doi:10.1016/j.drugpo.2015.05.002
World Heath Organisation. Appendix 3: systematic reviews and evidence summaries, PICO 1. Guidelines for the screening care and treatment of persons with chronic hepatitis c infection: updated version. WHO Guidelines Approved by the Guidelines Review Committee, Geneva; 2016
Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–88. doi:10.1056/NEJMoa1402355
U.S. Food and Drug Administration Approves Gilead’s Epclusa® (sofosbuvir/velpatasvir) for the treatment of all genotypes of chronic hepatitis c (internet); 2016. http://www.gilead.com/news/press-releases/2016/6/us-food-and-drug-administration-approves-gileads-epclusa-sofosbuvirvelpatasvir-for-the-treatment-of-all-genotypes-of-chronic-hepatitis-c. Cited 12 July 2016
European Association for Study of Liver, Association Latinoamericana para el Estudio del H. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–64. doi:10.1016/j.jhep.2015.04.006
Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53(6):1013–21. doi:10.1016/j.jhep.2010.05.035
Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 2014;20(10):2515–32. doi:10.3748/wjg.v20.i10.2515
Wiersma ST, McMahon B, Pawlotsky JM, Thio CL, Thursz M, Lim SG, et al. Treatment of chronic hepatitis B virus infection in resource-constrained settings: expert panel consensus. Liver Int. 2011;31(6):755–61. doi:10.1111/j.1478-3231.2010.02373.x
Sciences G. Viral hepatitis—developing world access. http://www.gilead.com/responsibility/developing-world-access/viralhepatitis. 12 July 2016
Freeman J, Sallie R, Kennedy A, Nieu PTN, Freeman J, Jeffreys G, et al. High sustained virological response rates using generic direct antiviral treatment for hepatitis C REDEMPTION-1 European Association for Study of Liver, Barcelona; 2016
European Association for Study of Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol. 2015;63(1):199–236. doi:10.1016/j.jhep.2015.03.025
Panel AIHG. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus 2016. http://www.hcvguidelines.org/full-report/when-and-whom-initiate-hcv-therapy. Cited 2016
Dieterich D, Bacon BR, Flamm SL, Kowdley KV, Milligan S, Tsai N, et al. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population. Washington DC: American Association of the Study of Liver Disease; 2014
Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594–603. doi:10.1056/NEJMoa1315722
Reddy KR, Bourliere M, Sulkowski M, Omata M, Zeuzem S, Feld JJ, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology. 2015;62(1):79–86. doi:10.1002/hep.27826
Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–21. doi:10.1056/NEJMoa1306218
Omata M, Kanda T, Yu M-L, Yokosuka O, Lim SG, Jafri W, et al. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int. 2012;6:409–35
Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867–77. doi:10.1056/NEJMoa1214854
Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Effectiveness of sofosbuvir-based regimens in genotype 1 and 2 hepatitis C virus infection in 4026 U.S. veterans. Aliment Pharmacol Ther. 2015;42(5):559–73. doi:10.1111/apt.13300
Gane EJ, Hyland R, Yang Y, Svarovskaia E, Pang PS, McHutchison JG, et al. Ledipasvir/sofosbuvir single tablet regimen is effective in patients with HCV genotype 2 infection international symposium on viral hepatitis and liver disease, Berlin; 2015
Foster GR, McLauchlan J, Irving W, Cheung M, Hudson B, Verma S, et al. Treatment of decompensated HCV cirrhosis in patients with diverse genotypes: 12 weeks sofosbuvir and NS5A inhibitors with/without ribavirin is effective in HCV genotypes 1 and 3. Vienna: European Association for Study of the Liver; 2015
Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61(4):1127–35. doi:10.1002/hep.27726
Hezode C, de Ledinghen V, Fontaine H, Zoulim F, Lebray P, Boyer N, et al. Daclatasvir plus sofosbuvir with or without ribavirin in patients with HCV genotype 3 infection: interim analysis of a French multicenter compassionate use program. Vienna: European Association for Study of the Liver; 2015
Doss W, Shiha G, Hassany M, Soliman R, Fouad R, Khairy M, et al. Sofosbuvir plus ribavirin for treating Egyptian patients with hepatitis C genotype 4. J Hepatol. 2015;63(3):581–5. doi:10.1016/j.jhep.2015.04.023
Abergel A, Metivier S, Samuel D, Jiang D, Kersey K, Pang PS, et al. Ledipasvir plus sofosbuvir for 12 weeks in patients with hepatitis C genotype 4 infection. Hepatology. 2016. doi:10.1002/hep.28706
Welzel TM, Herzer K, Ferenci P, Petersen J, Gschwantler M, Cornberg M, et al. Daclatasvir plus sofosbuvir with or without ribavirin for the treatment of HCV in patients with severe liver disease: interim results of a multicenter compassionate use program European Association for Study of Liver, Barcelona; 2015
Abergel A, Asselah T, Metivier S, Kersey K, Jiang D, Mo H, et al. Ledipasvir–sofosbuvir in patients with hepatitis C virus genotype 5 infection: an open-label, multicentre, single-arm, phase 2 study. Lancet Infect Dis. 2016;16(4):459–64. doi:10.1016/S1473-3099(15)00529-0
Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–87. doi:10.1056/NEJMoa1214853
Gane EJ, Hyland RH, An D, Svarovskaia E, Pang PS, Brainard D, et al. Efficacy of ledipasvir and sofosbuvir, with or without ribavirin, for 12 weeks in patients with HCV genotype 3 or 6 infection. Gastroenterology. 2015;149(6):1454–1461 e1. doi:10.1053/j.gastro.2015.07.063
Lai CL, Wong VW, Yuen MF, Yang JC, Knox SJ, Mo H, et al. Sofosbuvir plus ribavirin for the treatment of patients with chronic genotype 1 or 6 hepatitis C virus infection in Hong Kong. Aliment Pharmacol Ther. 2016;43(1):96–101. doi:10.1111/apt.13429
Organisation WH. Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva: WHO Guidelines Approved by the Guidelines Review Committee; 2014
Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373(27):2599–607. doi:10.1056/NEJMoa1512610
Foster GR, Afdhal N, Roberts SK, Brau N, Gane EJ, Pianko S, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373(27):2608–717. doi:10.1056/NEJMoa1512612
Nwokediuko SC. Chronic hepatitis B: management challenges in resource-poor countries. Hepat Mon. 2011;11(10):786–93. doi:10.5812/kowsar.1735143X.757
Tarn YH, Hu S, Kamae I, Yang BM, Li SC, Tangcharoensathien V, et al. Health-care systems and pharmacoeconomic research in Asia-Pacific region. Value Health. 2008;11(Suppl 1):S137–55. doi:10.1111/j.1524-4733.2008.00378.x
United Nations. Sixty-ninth session of the United National General Assembly: Draft outcome document of the United Nations summit for the adoption of the post-2015 development agenda. New York: United Nations; 2013. http://www.un.org.libproxy1.nus.edu.sg/ga/search/view_doc.asp?symbol=A/69/L.85&Lang=E.10 July 2016
Garrett L, Chowdhury AM, Pablos-Mendez A. All for universal health coverage. Lancet. 2009;374(9697):1294–9. doi:10.1016/S0140-6736(09)61503-8
Gupta V, Kerry VB, Goosby E, Yates R. Politics and universal health coverage—the post-2015 global health agenda. N Engl J Med. 2015;373(13):1189–92. doi:10.1056/NEJMp1508807
McKee M, Balabanova D, Basu S, Ricciardi W, Stuckler D. Universal health coverage: a quest for all countries but under threat in some. Value Health. 2013;16(1 Suppl):S39–45. doi:10.1016/j.jval.2012.10.001
Waked I, Doss W, El-Sayed MH, Estes C, Razavi H, Shiha G, et al. The current and future disease burden of chronic hepatitis C virus infection in Egypt. Arab J Gastroenterol. 2014;15(2):45–52. doi:10.1016/j.ajg.2014.04.003
El-Sayed MH. Egypt national programme for treatment of chronic HCV 2016. http://hcvhub.deusto.es/casestudy/egypt-national-programme-for-treatment-of-chronic-hcv. Cited 26 December 2016
Ford N, Singh K, Cooke GS, Mills EJ, von Schoen-Angerer T, Kamarulzaman A, et al. Expanding access to treatment for hepatitis C in resource-limited settings: lessons from HIV/AIDS. Clin Infect Dis. 2012;54(10):1465–72. doi:10.1093/cid/cis227
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was used for the preparation of the paper.
Conflict of interest
Seng Gee Lim received honoraria from Gilead Sciences, Merck Sharpe and Dohme, Bristol Myers Squibb, Abbvie, Abbott Diagnostics for advisory board duties and speaker duties, and has received research grants from Merck Sharpe and Dohme, Gilead Sciences and Abbott Diagnostics.
Ethical Compliance
None. No human subjects were enrolled for this manuscript.
Rights and permissions
About this article
Cite this article
Lim, S.G. HCV management in resource-constrained countries. Hepatol Int 11, 245–254 (2017). https://doi.org/10.1007/s12072-017-9787-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-017-9787-0