Abstract
Background
Historically, chronic hepatitis C virus (HCV) treatment was response-guided. Clinical trials with sofosbuvir indicated on-treatment virologic response was not predictive of sustained virologic response (SVR) and hence response-guided therapy (RGT) was abandoned. The purpose of this study is to examine the association between on-treatment 4-week HCV RNA and SVR in patients treated in real-world practice.
Methods
The study is a retrospective analysis of consecutive patients started on treatment with a sofosbuvir-containing regimen, January 1, 2014 through August 20, 2014, for HCV genotype 1–6 infection. Patients were treated by HCV specialists at 6 centers in the Project ECHO (Extension for Community Healthcare Outcomes) HCV Collaborative or in the community by primary care clinicians mentored by HCV specialists through Project ECHO. Patients were included if they were over 18 years, had evidence of chronic HCV, and were started on a sofosbuvir-containing regimen. The aspartate aminotransferase:platelet ratio index (APRI) was used to estimate fibrosis. The main outcome measures were 4-week HCV RNA and SVR.
Results
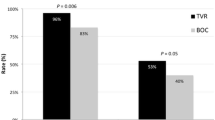
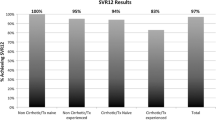
Overall SVR was 82.5 %. At week 4, HCV RNA was detected in 27.4 % of patients. Stepwise multivariable logistic-regression analyses identified APRI > 1.0, male sex, genotype 3, and detectable on treatment 4-week HCV RNA as independent predictors of failure to achieve SVR.
Conclusions
In a real-world setting, a significant proportion of sofosbuvir treated patients have detectable on-treatment 4-week HCV RNA. Detectable on-treatment 4-week HCV RNA is associated with virologic failure. More data are needed to formulate guidance for RGT with newly available HCV therapies.
Similar content being viewed by others
Abbreviations
- AASLD:
-
American Association for the Study of Liver Disease
- AST:
-
Aspartate transaminase
- APRI:
-
Aspartate transaminase platelet ratio index (APRI)
- BMI:
-
Body mass index
- C.I.:
-
Confidence interval
- DAAs:
-
Direct acting antiviral agents
- ECHO:
-
Extension for Community Healthcare Outcomes
- FDA:
-
United States Food and Drug Administration
- GT:
-
Genotype
- HCV:
-
Hepatitis C virus
- HCV RNA:
-
Hepatitis C virus ribonucleic acid
- HCC:
-
Hepatocellular carcinoma
- IAS-USA:
-
International Antiviral Society-USA
- IDSA:
-
Infectious Disease Society of America
- IFN:
-
Interferon
- PEG:
-
Pegylated interferon
- RBV:
-
Ribavirin
- RGT:
-
Response-guided therapy
- SD:
-
Standard deviation
- SMV:
-
Simeprevir
- SOF:
-
Sofosbuvir
- SVR:
-
Sustained virologic response
- UNMHSC:
-
University of New Mexico Health Sciences Center
References
van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012;308(24):2584–2593
Johnson RJ, Gretch DR, Yamabe H, et al. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 1993;328(7):465–470
Younossi ZM, Stepanova M, Gerber L, Nader F, Frost S, Hunt SL. P717 Improvement of central fatigue is associated with sustained virologic response (SVR) following sofosbuvir (SOF) containing regimens. J Hepatol 2014;60(1):S308
Arase Y, Suzuki F, Suzuki Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology 2009;49(3):739–744
Takahashi K, Nishida N, Kawabata H, Haga H, Chiba T. Regression of Hodgkin lymphoma in response to antiviral therapy for hepatitis C virus infection. Intern Med 2012;51(19):2745–2747
Lawitz EJ, Membreano FE. Response-guided therapy in patients with genotype 1 hepatitis C virus: Current status and future prospects. J Gastroenterol Hepatol 2014;29(8):1574–1581
Fried MW, Hadziyannis SJ, Shiffman ML, et al. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol 2011;55:69–75
Lawitz, E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878–1887
Arora S, Thornton K, Komaromy M, et al. Demonopolizing medical knowledge. Academic Medicine 2014;89(1):30–32
Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment—Extension for Community Healthcare Outcomes (ECHO) project: Disruptive innovation in specialty care. Hepatology 2010;52(3):1123–1133
Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 2011;364:2199–2207
Lin ZH, Xin YN, Dong QJ, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011;53:726–36
Taylor N, Haschke-Becher E, Greil R, et al. Performance characteristics of the COBAS Ampliprep/COBAS TaqMan v2.0 and the Abbott RealTime hepatitis C assays—implications for response-guided therapy in genotype 1 infections. Antiviral Ther 2014;19(5):449–454
Sidharthan S, Kohli A, Sims Z, et al. Utility of hepatitis C viral load monitoring on direct-acting antiviral therapy. Clin Infect Dis 2015;60(12):1743–1751
Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. New Engl J Med 2013;368(20):1867–1877
Dieterich D, Bacon B, Flamm SL et al. Evaluation of sofosbuvir and simeprevir-based regimens in the TRIO network: academic and community treatment of a real-world, heterogeneous population. [Abstract 46.] 65th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD). 2014a;220A; Boston, MA
Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. New Engl J Med 2014;370:1483–1493
Kohli A, Osinusi A, Sims Z, et al. Virologic response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study. Lancet 2015;385:1107–1113
American Association for the Study of Liver Diseases. Recommendations for testing, managing, and treating Hepatitis C. [Internet]. Arlington (VA): doi:10.1002/hep.27950. Available from: http://www.hcvguidelines.org/. Jointly published with Infectious Diseases Society of America
Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. New Engl J Med 2014;370:1483–1493
Bourliere M, Sulkowski M, Omata M, et al. An integrated safety and efficacy analysis of > 500 patients with compensated cirrhosis treated with ledipasvir/sofosbuvir with or without ribavirin. 65th Annual Meeting of the American Association for the Study of Liver Diseases; 2014; Boston, MA
Poordad F, Hezode C, Trinh R, et al. ABT-450/r–ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. New Engl J Med 2014;370:1973–1982
Simeprevir [package insert]. Titusville NJ: Janssen Therapeutics, 2013
Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet 2014;384:1756–1765
Lawitz E, Flamm S, Yang JC, et al. Retreatment of patients who failed 8 or 12 weeks of ledipasvir/sofosbuvir-based regimens with ledipasvir/sofosbuvir for 24 weeks. 50th Annual Meeting of the European Association for the Study of the Liver; 2015; Vienna
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Karla Thornton, MD, MPH No financial, personal or professional conflicts of interest to disclose. Paulina Deming, PharmD No financial, personal or professional conflicts of interest to disclose. Richard A. Manch, MD, FAASLD, FACP, FACG Speaker and/or consultant for Gilead, Janssen, and AbbVie. Ann Moore, FNP Speaker and/or consultant for Gilead, AbbVie, BMS, Merck Anita Kohli, MD, MS No financial, personal or professional conflicts of interest to disclose. Robert Gish, MD Research support from Bristol-Myers Squibb Company, Gilead, Merck, Benitec, AbbVie. Consultant a/o Advisor for Abbot, AbbVie, Arrowhead, Bayer AG, Bristol-Myers, Squibb Company, Contravir, Eiger, Enyo, Genentech, Gilead Sciences, Hoffmann-LaRocher Ltd., Hologic, Idenix, Intercept, Isis Pharmaceuticals, Janssen, MedImmune, Merck, NittoDenko, Onyx. Clinical Advisory Board for AbbVie, Merck, Arrowhead, Contravir, Novira, Intercept, Isis, Persidio, Eiger, Enyo, Janssen, Medimune. Chair of Clinical Advisory board for Arrowhead. Data safety monitoring board for Novira and Presidio. Consulting confidentiality agreements with Intercept (2010–2015), Contravir (2015–present), Genentech (2015–present), Tekmira (2011–2015), Janssen (2015–present), MedImmune (2015–present), Eiger (2015–present), Novira (2015–present), Presidio (2015–present). Speaker’s bureau for: Bayer, BMS, Gilead Sciences Inc., Salix/Valeant, AbbVie. Minor stock shareholder for Kinex, Synageva, RiboSciences, CoCrystal. Stock options for Arrowhead. Norman Sussman, MD, FAASLD Research for AbbVie, BMS, Merck, Gilead. Consulting for Merck, BMS. Speaker for AbbVie, Janssen, Gilead. Saira Khaderi, MD No financial, personal or professional conflicts of interest to disclose. John Scott, MD, MSc Advisory board for Gilead, BMS. Data safety monitoring board for Tacere Therapeutics. Funding for clinical trial from Merck. Jorge Mera, MD No financial, personal or professional conflicts of interest to disclose. Terry Box, MD Advisory board for AbbVie, Gilead, Janssen. Funding from AbbVie, Gilead, Conatus, BMS, Boehringer, Ingelheim, Ikaria, Cumberland, Sundise, Salix, Novartis, Genfit. Speaker for AbbVie, Gilead, Janssen, Salix. Clifford Qualls, PhD No financial, personal or professional conflicts of interest to disclose. Miranda Sedillo, MS No financial, personal or professional conflicts of interest to disclose. Sanjeev Arora, MD Funding for research grants: Gilead, Merck, AbbVie.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not obtained because the study was retrospective and observational.
Writing assistance
There are no additional contributors to acknowledge outside the authors listed above.
Grant Support
This research project was not funded.
Rights and permissions
About this article
Cite this article
Thornton, K., Deming, P., Manch, R.A. et al. Is response guided therapy dead? Low cure rates in patients with detectable hepatitis C virus at week 4 of treatment. Hepatol Int 10, 624–631 (2016). https://doi.org/10.1007/s12072-016-9725-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-016-9725-6