Abstract
Purpose
A randomized, double-blind, multicenter study (ETV-047) was conducted to evaluate the dose–response relationship of entecavir and compare its antiviral activity and safety with lamivudine in Japanese patients with chronic hepatitis B (CHB).
Methods
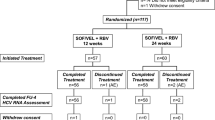
One hundred thirty-seven nucleoside-naive adult patients with CHB were randomized to once-daily oral doses of entecavir 0.01, 0.1, or 0.5 mg or lamivudine 100 mg for 24 weeks. The primary efficacy end point used to evaluate the dose–response relationship was mean change from baseline in serum hepatitis B virus (HBV) DNA level at week 22, as determined by polymerase chain reaction assay.
Results
Entecavir demonstrated a clear dose–response relationship, with mean change from baseline in serum HBV DNA level of −3.11, −4.77, and −5.16 log10 copies/ml with entecavir 0.01, 0.1, and 0.5 mg, respectively. Entecavir 0.5 mg was superior to lamivudine 100 mg for the mean change in HBV DNA level (−5.16 vs. −4.29 log10 copies/ml; P = 0.007). The overall incidence of adverse events was comparable between treatment groups. Two patients discontinued treatment because of adverse events (one with liver cirrhosis [entecavir 0.5 mg] and one with grade 4 serum alanine aminotransferase (ALT) elevation, nausea, and malaise [lamivudine 100 mg]). Serum ALT flares were observed in four patients; flares were associated with 2 log10 reductions or more in HBV DNA level and resolved without dose interruption.
Conclusion
Entecavir 0.01–0.5 mg is well tolerated and produces a dose-dependent reduction in viral load in nucleoside-naive Japanese patients with CHB. Compared with lamivudine 100 mg, entecavir 0.1 mg demonstrated noninferiority and entecavir 0.5 mg was superior in this population.

Similar content being viewed by others
References
Maddrey WC. Hepatitis B: an important public health issue. J Med Virol 2000;61:362–366. doi:10.1002/1096-9071(200007)61:3<362::AID-JMV14>3.0.CO;2-I
Kao JH, Chen DS. The natural history of hepatitis B virus infection. In Lai CL, Locarnini S, editors. Hepatitis B Virus. London: International Medical Press; 2002. 161–172
Lok AS, McMahon BJ. American Association for the Study of Liver Diseases (AASLD) practice guidelines. Chronic Hepatitis B. Hepatology 2007;45:507–539. doi:10.1002/hep.21513
Rivkin A. A review of entecavir in the treatment of chronic hepatitis B infection. Curr Med Res Opin 2005;21:1845–1856. doi:10.1185/030079905X65268
Wong DKH, Cheung AM, O’Rourke K, Naylor CD, Detsky AS, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med 1993;119:312–323
Allen MI, Deslauriers M, Andrews CW, Tipples GA, Walters KA, Tyrrell DL, et al. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Lamivudine Clinical Investigation Group. Hepatology 1998;27:1670–1677. doi:10.1002/hep.510270628
Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, et al. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol 2004;19(11):1276–1282. doi:10.1111/j.1440-1746.2004.03428.x
Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004;351(15):1521–1531. doi:10.1056/NEJMoa033364
Omata M. Treatment of chronic hepatitis B infection. N Engl J Med 1998;339:114. doi:10.1056/NEJM199807093390209
Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int 2008;2:263–283. doi:10.1007/s12072-008-9080-3
Genovesi EV, Lamb L, Medina I, Taylor D, Seifer M, Innaimo S, et al. Efficacy of the carbocyclic 2′-deoxyguanosine nucleoside BMS-200475 in the woodchuck model of hepatitis B virus infection. Antimicrob Agents Chemother 1998;42:3209–3217
Marion PL, Salazar FH, Winters MA, Colonno RJ. Potent efficacy of entecavir (BMS-200475) in a duck model of hepatitis B virus replication. Antimicrob Agents Chemother 2002;46:82–88. doi:10.1128/AAC.46.1.82-88.2002
Colonno RJ, Rose R, Baldick CJ, Levine S, Pokornowski K, Yu CF, et al. Entecavir resistance is rare in nucleoside naïve patients with hepatitis B. Hepatology 2006;44:1656–1665. doi:10.1002/hep.21422
Ono SK, Kato N, Shiratori Y, Kato J, Goto T, Schinazi RF, et al. The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J Clin Invest 2001;107:449–455. doi:10.1172/JCI11100
Lai CL, Rosmawati M, Lao J, Van Vlierberghe H, Anderson FH, Thomas N, et al. Entecavir is superior to lamivudine in reducing hepatitis B virus DNA in patients with chronic hepatitis B infection. Gastroenterology 2002;123:1831–1838. doi:10.1053/gast.2002.37058
Chang TT, Gish RG, Hadziyannis SJ, Cianciara J, Rizzetto M, Schiff ER, et al. A dose-ranging study of the efficacy and tolerability of entecavir in lamivudine-refractory chronic hepatitis B patients. Gastroenterology 2005;129:1198–1209. doi:10.1053/j.gastro.2005.06.055
Ling R, Mutimer D, Ahmed M, Boxall EH, Elias E, Dusheiko GM, et al. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology 1996;24:711–713. doi:10.1002/hep.510240339
Tipples GA, Ma MM, Fischer KP, Bain VG, Kneteman NM, Tyrrell DLJ. Mutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology 1996;24:714–717
Tenney DJ, Levine SM, Rose RE, Walsh AW, Weinheimer SP, Discotto L, et al. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to lamivudine. Antimicrob Agents Chemother 2004;48:3498–3507. doi:10.1128/AAC.48.9.3498-3507.2004
Chang TT, Gish R, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006;354:1074–1076. doi:10.1056/NEJMoa051285
Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw Y-F, Cianciara J, et al. Entecavir is superior to continued lamivudine for the treatment of lamivudine-refractory. HBeAg-positive chronic hepatitis B. Gastroenterology 2006;130:2039–2049
Boni C, Bertoletti A, Penna A, Cavalli A, Pilli M, Urbani S, et al. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J Clin Invest 1998;102:968–975. doi:10.1172/JCI3731
Nair S, Perrillo RP. Serum alanine aminotransferase flares during interferon treatment of chronic hepatitis B: is sustained clearance of HBV DNA dependent on levels of pretreatment viremia? Hepatology 2001;34:1021–1026. doi:10.1053/jhep.2001.28459
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shindo, M., Chayama, K., Mochida, S. et al. Antiviral activity, dose–response relationship, and safety of entecavir following 24-week oral dosing in nucleoside-naive Japanese adult patients with chronic hepatitis B: a randomized, double-blind, phase II clinical trial. Hepatol Int 3, 445–452 (2009). https://doi.org/10.1007/s12072-009-9135-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-009-9135-0