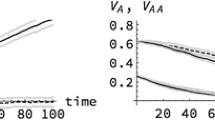
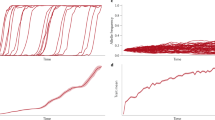
Abstract
Although molecular methods, such as QTL mapping, have revealed a number of loci with large effects, it is still likely that the bulk of quantitative variability is due to multiple factors, each with small effect. Typically, these have a large additive component. Conventional wisdom argues that selection, natural or artificial, uses up additive variance and thus depletes its supply. Over time, the variance should be reduced, and at equilibrium be near zero. This is especially expected for fitness and traits highly correlated with it. Yet, populations typically have a great deal of additive variance, and do not seem to run out of genetic variability even after many generations of directional selection. Long-term selection experiments show that populations continue to retain seemingly undiminished additive variance despite large changes in the mean value. I propose that there are several reasons for this. (i) The environment is continually changing so that what was formerly most fit no longer is. (ii) There is an input of genetic variance from mutation, and sometimes from migration. (iii) As intermediate-frequency alleles increase in frequency towards one, producing less variance (as p → 1, p(1 − p) → 0), others that were originally near zero become more common and increase the variance. Thus, a roughly constant variance is maintained. (iv) There is always selection for fitness and for characters closely related to it. To the extent that the trait is heritable, later generations inherit a disproportionate number of genes acting additively on the trait, thus increasing genetic variance. For these reasons a selected population retains its ability to evolve. Of course, genes with large effect are also important. Conspicuous examples are the small number of loci that changed teosinte to maize, and major phylogenetic changes in the animal kingdom. The relative importance of these along with duplications, chromosome rearrangements, horizontal transmission and polyploidy is yet to be determined. It is likely that only a case-by-case analysis will provide the answers. Despite the difficulties that complex interactions cause for evolution in Mendelian populations, such populations nevertheless evolve very well. Longlasting species must have evolved mechanisms for coping with such problems. Since such difficulties do not arise in asexual populations, a comparison of epistatic patterns in closely related sexual and asexual species might provide some important insights.
Similar content being viewed by others
References
Bulmer M. G. 1980 The mathematical theory of quantitative genetics. The Clarendon Press, Oxford.
Carlborg O. and Haley C. 2008 Epistasis: too often neglected in complex trait studies. Nat. Rev. Genet. 5, 618–625.
Carroll S. B. 2006 The making of the fittest. W. W. Norton, New York.
Cockerham C. C. 1954 An extension of the concept of partitioning hereditary variance for analysis of covariance among relatives when epistasis is present. Genetics 39, 859–882.
Crow J. F. 1957 Genetics of DDT resistance in Drosophila. Proc. Intern. Genet. Symp. Jpn. 1956, 408–409.
Crow J. F. 1992 An advantage of sexual reproduction in a rapidly changing environment. J. Hered. 83, 169–173.
Crow J. F. and Kimura M. 1970 An introduction to population genetics theory. Burgess, Minneapolis.
Crow J. F. and Kimura M. 1979 Efficiency of truncation selection. Proc. Natl. Acad. Sci. USA 76, 396–399.
Dudley J. W. 2007 From means to QTL: The Illinois long-term selection experiment as a case study in quantitative genetics. Crop Sci. 47, S20–S31.
Fisher R. A. 1918 The correlation between relatives on the supposition of Mendelian inheritance. Proc. R. Soc. Edin. 52, 399–433.
Fisher R. A. 1930 The genetical theory of natural selection. The Clarendon Press Oxford, (Variorum edition 1999, Oxford University Press, Oxford).
Fisher R. A. 1941 Average excess and average effect of a gene subsitution. Ann. Eugen. 11, 53–63.
Greenberg R. and Crow J. F. 1960 A comparison of lethal and detrimental chromosomes from natural populations. Genetics 45, 1153–1168.
Hamilton W. D., Axelrod A. and Tanese R. 1990 Sexual reproduction as an adaptation to resist parasites (a review). Proc. Natl. Acad. Sci. USA 87, 3566–3573.
Hill W. G. 1982 Predictions of response to artificial selection from new mutations. Genet. Res. 40, 256–278.
Hill W. G. 2005 A century of corn selection. Science 307, 683–684.
Hill W. G., Goddard M. E. and Visscher P. M. 2008 Data and theory point to mainly additive genetic variance for complex traits. PLoS Genet. 4, e1000008.
Kimura M. 1965 Attainment of quasi-linkage equilibrium when gene frequencies are changing by natural selection. Genetics 52, 875–890.
Kondrashov A. S. 1982 Selection against harmful mutations in large sexual and asexual populations. Genet. Res. 26, 221–235.
Laurie C. C., Chasalow S. D., Le Deaux J. R., McCarroll R., Bush D., Hauge B. et al. 2004 The genetic architecture of response to long-term artificial selection for oil concentration in the maize kernel. Genetics 168, 2141–2155.
Malmberg R. 1977 The evolution of epistasis and the advantage of recombination in populations of bacteriophage T4. Genetics 86, 607–621.
Robertson A. 1955 Selection in animals: synthesis. Cold Spring Harbor Symp. Quant. Biol. 20, 225–229.
Shao H., Burrage L. C., Sinasac D. S., Hill A. E., Ernest S. R., O’Brien W. O. et al. 2008 Genetics architecture of complex traits: large phenotypic effects and pervasive epistasis. Proc. Natl. Acad. Sci. USA (in press).
Sved J. A. 1968 Possible rates of gene substitution in evolution. Am. Nat. 102, 283–293.
Temin R. G., Meyer H. U., Dawson P. S. and Crow J. F. 1969 The effect of epistasis on homozygous viability depression in Drosophila melanogaster. Genetics 61, 497–519.
Visscher P. M. 2008 Sizing up human height variation. Nat. Genet. 40, 489–490.
Wright S. 1965 Factor interaction and linkage in evolution. Proc. R. Soc. London. B 162, 80–104.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crow, J.F. Maintaining evolvability. J Genet 87, 349–353 (2008). https://doi.org/10.1007/s12041-008-0057-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-008-0057-8