Abstract
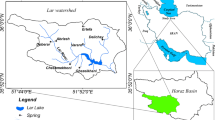
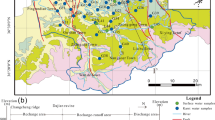
Water samples were collected from precipitation, streams and karst springs of the mountainous Bringi catchment of Kashmir Himalayas for major ions, stable isotopes (δ 18O and δD) and 3H analysis. The main objective is to identify the potential recharge area for karst springs. The water in the Triassic limestone aquifer of the Bringi watershed is characterized by low levels of mineralization with TDS of the spring water samples ranging between 99 and 222 mg/l except the Kongamnag spring, which contained TDS up to 425 mg/l. As expected in an area with dominant carbonate lithology, Ca–HCO 3 and Ca–Mg–HCO 3 hydrochemical facies were found. Based on the amount weighed monthly averages (n = 6), the local meteoric water line (LMWL) for Bringi watershed is δD = 7.7 ×δ 18O + 11.1 (r 2 = 0.99). The isotopic signature of winter precipitation is reflected in stream and spring water in late spring and is therefore, a representative of snow melting. The spring waters in September bear the δ 2H and δ 18O enriched isotopic signatures of summer rainfall. With the help of the local vertical isotopic gradient of precipitation (δ 18O=−0.27‰ per 100 m increase in elevation), the mean elevation of precipitation that recharged the aquifer is estimated and ranges about 2500–2900 m amsl. There is a very strong correlation (r 2 = 0.97) between the seasonal isotope composition of streams and springs, indicating that streams and springs either share similar catchments or the springs are recharged by the streams.








Similar content being viewed by others
References
Abbott M D, Lini A and Bierman P R 2000 δ 18O, δD and 3H measurements constrain groundwater recharge patterns in an upland fractured bedrock aquifer, Vermont, USA; J. Hydrol. 228 101–112.
Agarwal M, Gupta S K, Deshpande R D and Yadava M G 2006 Helium, radon and radiocarbon studies on a regional aquifer system of the north Gujarat–Cambay region, India; Chem. Geol. 228 209–232.
APHA 1998 Standard methods for the examination of water and waste; American Public Health Association, Washington DC.
Bhat N A, Jeelani G. and Bhat M Y 2014 Hydrogeochemical assessment of groundwater in karst environments, Bringi watershed, Kashmir Himalayas, India; Curr. Sci. 106 (7) 1000–1007.
Clark I D and Fritz P 1997 Environmental isotopes in hydrogeology; Lewis Publishers, Boca Raton.
Coward J M H, Waltham A C and Bowser R J 1972 Karst springs in the Vale of Kashmir; J. Hydrol. 16 213– 223.
Craig H 1961 Isotopic variation in meteoric waters; Science 133 1702–1703.
Dansgaard W 1964 Stable isotopes in precipitation; Tellus 16 436–467.
Epstein S and Mayeda T 1953 Variation of δ 18O content in waters from natural sources; Geochim. Cosmochim. Acta 4 213–224.
Fontes J C, Letolle R, Oliver H and Ravoux B 1967 Oxygene 18 et tritium dans le basin d’Evian; In: Proceedings of symposium on isotopes in hydrology, International Atomic Energy Agency, Vienna, 401p.
Ford D and Williams P 1989 Karst Geomorphology and Hydrology; London: Chapman and Hall.
Fredrickson G C and Criss R E 1999 Isotope hydrology and time constants of the unimpounded Meramec River basin, Missouri; Chem. Geol. 157 303–317.
Gat J R 1971 Comments on the stable isotope method in regional groundwater investigations; Water Resourc. Res. 7 980–993.
Gonfiantini R 1981 The δ-notation and the mass spectrometric measurement techniques; In: Stable isotope hydrology, deuterium and oxygen in the water cycle, International Atomic Energy Agency, Vienna.
Gonfiantini R, Gallo G, Payne B R and Taylor C B 1976 Environmental isotopes and hydrochemistry in groundwater of Gran Canaria; In: Interpretation of environmental isotope and hydrochemical data in groundwater hydrology, IAEA, Vienna, pp. 159–170.
Ingraham N L and Taylor B E 1991 Light stable isotope systematic of large-scale hydrologic regimes in California and Nevada; Water Resourc. Res. 27 77–90.
Jeelani G 2008 Aquifer response to regional climate variability in a part of Kashmir Himalaya in India; Hydrogeol. J. 16 1625–1633.
Jeelani G, Bhat N A and Shivanna K 2010 Use of δ 18O tracer to identify stream and spring origins of a mountainous catchment: A case study from Liddar watershed, western Himalaya, India; J. Hydrol. 393 257–264.
Jeelani G, Bhat N A, Shivanna K and Bhat M Y 2011 Geochemical characterization of surface water and spring water in SE Kashmir valley, western Himalayas: Implication to water–rock interaction; J. Earth Syst. Sci. 120 (5) 921–932.
Jeelani G, Kumar S U, Bhat N A, Sharma S and Kumar B 2014 Variation of δ 18O, δD and 3H in karst springs of south Kashmir, western Himalayas (India); Hydrol. Process., doi: 10.1002/hyp.10162.
Kattan Z 1997 Environmental isotope study of the major karst springs in Damuscus limestone aquifer system: Case of the Figeh and Barada springs; J. Hydrol. 193 161– 182.
Kumar B, Rai S P, Kumar U S, Verma S K, Garg P, Kumar S V V, Jaiswal R, Purendra B K, Kumar S R and Pande N G 2010 Isotopic characteristics of Indian precipitation; Water Resourc. Res. 46 1–15.
Lee K S, Wenner D B and Lee I 1999 Using H- and O-isotopic data for estimating the relative contributions of rainy and dry season precipitation to groundwater: Example from Cheju Island, Korea; J. Hydrol. 222 65–74.
Longinelli A and Selmo E 2003 Isotopic composition of precipitation in Italy: a first overall map; J. Hydrol. 270 75–88.
McConville C, Kalin R M, Johnston H and McNeil G W 2001 Evaluation of recharge in a small temperate catchment using natural and applied δ 18O profiles in the unsaturated zone; Ground Water 39 616–624.
Middlemiss C S 1910 A revision of Silurian–Triassic sequence of Kashmir; Rec. Geol. Surv. India 40 206–260.
O’Driscoll M A, DeWalle D R, McGuire K J and Gburek W J 2005 Seasonal 18O variations and groundwater recharge for three landscape types in central Pennsylvania, USA; J. Hydrol. 303 108–124.
Price R M and Swart P K 2006 Geochemical indicators of groundwater recharge in the surficial aquifer system, Everglades National Park, Florida, USA; In: Perspectives on karst geomorphology, hydrology, and geochemistry – A tribute volume to Derek C Ford and William B White. (eds) Harmon R S and Wicks C, Geol. Soc. Am. Spec. Paper 404 251–266.
Rozanski K, Aruguás-Aruguás L and Ganfiantini R 1993 Isotopic patterns in modern global precipitation; Geophys. Monograph 78 1–36.
Siegenthaler U and Oeschger H 1980 Correlation of 18O in precipitation with temperature and altitude; Nature 285 314–317.
Smith D I 1993 The nature of karst aquifers and their susceptibility to pollution; Catena Suppl. 25 41–58.
Smith G I, Friedman I, Klieforth H E and Hardcastle K 1979 Aerial distribution of deuterium in eastern California precipitation; J. Appl. Meteorol. 18 172–188.
Wadia D N 1975 Geology of India; Tata McGraw Hill, New Delhi.
Yehdegho B and Reichl P 2002 Recharge areas and hydrochemistry of carbonate springs issuing from Semmering Massif, Austria, based on long-term oxygen-18 and hydrochemical data evidence; Hydrogeol. J. 10 628– 642.
Acknowledgements
The authors thank the scientists and staff at HIS, IAD, BARC, Mumbai and NIH, Roorkee for mass spectrometric analysis. The financial support from Board of Research in Nuclear Science (BRNS), Department of Atomic Energy, Government of India, is gratefully acknowledged. The comments and suggestions of the anonymous reviewers are highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
BHAT, N.A., JEELANI, G. Delineation of the recharge areas and distinguishing the sources of karst springs in Bringi watershed, Kashmir Himalayas using hydrochemistry and environmental isotopes. J Earth Syst Sci 124, 1667–1676 (2015). https://doi.org/10.1007/s12040-015-0629-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12040-015-0629-y