Abstract
An energy cassette, N-butyl-6-(3-phenylethynyl-1,3,7,9-tetramethylBODIPY)-naphthalimide (BON), was constructed with an energy donor and acceptor incorporated in a single molecular system. The energy donor (naphthalimide, NA) and acceptor (BODIPY) were bonded through phenylacetylene, a conjugate linker. Highly twisted molecular conformation was formed due to intramolecular repulsion, forcing the two fluorophores to act as independent chromophores. Therefore, the absorption spectra of BON in a common organic solvent are of superimposition of NA and BODIPY. Upon excitation of UV light (365 nm), a typical BODIPY emission character was observed, indicating an efficient energy transfer from NA moiety to the BODIPY scaffold with a transfer efficiency of 98%. Additionally, the pseudo-Stokes’ shift expanded to around 140 nm, which is largely longer than traditional BODIPY dyes (~ 10 nm). The frontier molecular orbitals analysis shows that there exists a pronounced electron density shifting from HOMO to LUMO, suggesting the efficient energy transfer in BON. A through-space energy transfer cassette was established with the twisted molecular conformation-induced orbital decoupling and favorable mutual orientation of the excited NA/BODIPY moment vector.
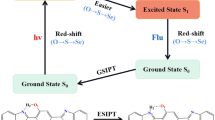
Graphical abstract
A dye pair (BON) was configured by naphthalimide and BOIDPY with a conjugate linker, phenylacetylene. Due to intramolecular repulsion in BON, the dye molecule is highly twisted, forming an efficient energy transfer cassette. An efficient energy transfer from NA to BODIPY occurred with a transfer efficiency of 98%.










Similar content being viewed by others
References
Haick H and Tang N 2021 Artificial intelligence in medical sensors for clinical decisions ACS Nano 15 3557
Bello O and Zeadally S 2016 Intelligent device-to-device communication in the internet of things IEEE Syst. J. 10 1172
Hsu Y L, Chou P H, Chang H C, Lin S L, Yang S C, Su H Y, et al. 2017 Design and implementation of a smart home system using multisensor data fusion technology Sensors 17 1631
Su M and Song Y 2022 Printable smart materials and devices: strategies and application Chem. Rev. 122 5144
Gardan J 2019 Smart materials in additive manufacturing: state of the art and trends Virtual Phys. Prototyp. 14 1
Yu X, Cheng H, Zhang M, Zhao Y, Qu L and Shi G 2017 Graphene-based smart materials Nat. Rev. Mater. 2 17046
Qiu X and Hu S 2013 Smart materials based on cellulose: a review of the preparations, properties, and applications Materials 6 738
Stoppa M and Chiolerio A 2014 Wearable electronics and smart textiles: a critical review Sensors 14 11957
Li X, Zou Y, Heo G and Son Y A 2020 Emission shift of an imidazole bridged diethylaminocoumarin and diphenyl Mol. Cryst. Liq. Cryst. 704 48
Sheng Y, Ma J, Liu S, Wang Y, Zhu C and Cheng Y 2016 Strong and reversible circularly polarized luminescence emission of a chiral 1,8-naphthalimide fluorophore induced by excimer emission and orderly aggregation Chem. Eur. J. 22 9519
Georgiev N I, Dimitrova M D, Todorova Y D and Bojinov V B 2016 Synthesis, chemosensing properties and logic behaviour of a novel ratiometric 1,8-naphthalimide probe based on ICT and PET Dyes Pigm. 131 9
Mishra A, Behera R K, Behera P K, Mishra B K and Behera G B 2000 Cyanines during the 1990s: a review Chem. Rev. 100 1973
Li X, Han Y, Min K and Son Y A 2018 Configuration of white light emission by coumarin and naphthalimide Mol. Cryst. Liq. Cryst. 660 10
Ulrich G, Ziessel R and Harriman A 2008 The Chemistry of fluorescent bodipy dyes: versatility unsurpassed Angew Chem. Int. Ed. 47 1184
Li X, Han Y, Sun S, Shan D, Ma X, He G, et al. 2020 A diaminomaleonitrile-appended BODIPY chemosensor for the selective detection of Cu2+ via oxidative cyclization and imaging in SiHa cells and zebrafish Spectrochim. Acta Part A 233 118179
Kenry Tang B Z and Liu B 2020 Catalyst: aggregation-induced emission—how far have we come, and where are we going next? Chem. 6 1195
Peng Q and Shuai Z 2021 Molecular mechanism of aggregation-induced emission Aggregate 2 e91
Mei J, Leung NLC, Kwok RTK, Lam JWY and Tang BZ 2015 Aggregation-induced emission: together we shine, united we soar! Chem. Rev. 115 11718
Mie J, Hong Y, Lam JWY, Qin A, Tang Y and Tang BZ 2014 Aggregation-induced emission: the whole is more brilliant than the parts Adv. Mater. 26 5429
Chen W, Chen C L, Zhang Z, Chen Y C, Chao W L, Su J, et al. 2017 Snapshotting the excited-state planarization of chemically locked N, N’-disubstituted dihydrodibenzo[a, c] phenazines J. Am. Chem. Soc. 139 1636
Sun G, Wei YC, Zhang Z, Lin JA, Liu ZY, Chen W, et al. 2020 Diversified excited-state relaxation pathways of donor-linker-acceptor dyads controlled by a bent-to-planar motion of the donor Angew. Chem., Int. Ed. 59 18611
Li X, Li F and Ji G 2023 A fluorescent turn-on sensor toward multiple heavy metal ions based on meso-anisole modified BODIPY scaffold J. Fluoresc. 33 631
Li X, Guo X, Chen Y, Cui T and Xing L 2021 Double 3-ethyl-2,4-diemthylpyrrole configured fluorescent dye with fluorine-boron as the bridge J Fluoresc. 31 1797
Loudet A and Burgess K 2007 BODIPY dyes and their derivatives: synthesis and spectroscopic properties Chem. Rev. 107 4891
Li X, Han Y, Kim M J and Son Y A 2018 A BODIPY-based highly emissive dye with thiophene-based branch harvesting the light Mol. Cryst. Liq. Cryst. 662 157
Tamgho I S, Hasheminasab A, Engle J T, Nemykin V N and Ziegler C J 2014 A new highly fluorescent and symmetric pyrrole-BF2 chromophore: BOPHY J. Am. Chem. Soc. 136 5623
Boodts S, Fron E, Hofkens H and Dehaen W 2018 The BOPHY fluorophore with double boron chelation: synthesis and spectroscopy Coordin. Chem. Rev. 371 1
Schmitt A, Hinkeldey B and Wild M 2009 Synthesis of the core compound of the BODIPY dye class: 4,4′-difluoro-4-bora-(3a,4a)-diaza-s-indacene J. Fluoresc. 19 755
Yoshii R, Yamane H, Nagai A, Tanaka K, Taka H, Kita H and Chujo Y 2014 π-Conjugated polymers composed of BODIPY or aza-BODIPY derivatives exhibiting high electron mobility and low threshold voltage in electron-only devices Macromolecules 47 2316
Rousseau T, Cravino A, Bura T, Ulrich G, Ziessel R and Roncali J 2009 BODIPY derivatives as donor materials for bulk heterojunction solar cells Chem. Commun. 13 1673
Olivier J H, Camerel F, Ulrich G, Barberá J and Ziessel R 2010 Luminescent ionic liquid crystals from self-assembled BODIPY disulfonate and imidazolium frameworks Chem. Eur. J. 16 7134
Aguiar A, Farinhas J, da Silva W, Susano M, Silva MR, Alcacer L, et al. 2020 Simple BODIPY dyes as suitable electron-donors for organic bulk heterojunction photovoltaic cells Dyes Pigm. 172 107842
Zhu H, Fan J, Wang J, Mu H and Peng X 2014 An enhanced PET based fluorescent probe with ultrasensitivity for imaging basal and elesclomol-induced HClO in cancer cells J. Am. Chem. Soc. 136 12820
Li X, Liao M, Sun J, Heo G and Son Y A 2019 Thiophene modulated BODIPY dye as a light harvester Mol. Cryst. Liq. Cryst. 679 127
Zhang Z, Zhang H, Jiao C, Ye K, Zhang H, Zhang J and Wang Y 2015 2-(2-Hydroxyphenyl)benzimidazole-based four-coordinate boron-containing materials with highly efficient deep-blue photoluminescence and electroluminescence Inorg. Chem. 54 2652
Li X, Tian G, Shao D, Xu Y, Wang Y, Ji G, et al. 2020 A BODIPY based emission signal turn-on probe toward multiple heavy metals Mol. Cryst. Liq. Cryst. 706 38
Li X, Zhou Q, Heo G and Son Y A 2018 2,4-Dimethylpyrrole configured fluorine-boron complexes Mol. Cryst. Liq. Cryst. 677 34
Dwivded B K, Singh R S, Ali A, Sharma V, Mobin S M and Pandey D S 2019 AIE active piperazine appended naphthalimide-BODIPYs: photophysical properties and applications in live cell lysosomal tracking Analyst 144 331
Eserci H, Çetin M, Aydinoglu F, Eçik E T and Okutan E 2022 Naphthalimide-BODIPY dyads: synthesis, characterization, photophysical properties, live cell imaging and antimicrobial effect J. Mol. Struct. 1265 133440
Poddar M, Sharma V, Mobin S M and Misra R 2018 1,8-Naphthalimide substituted BODIPY dyads: synthesis, structure, properties and live-cell imaging Chem. Asian J. 13 2881
Carlotti B, Poddar M, Elisei F, Spalletti A and Misra R 2019 Energy-transfer and charge-transfer dynamics in highly fluorescent naphthalimide−BODIPY dyads: effect of BODIPY orientation J. Phys. Chem. C 123 24362
Mukherjee S and Thilagar P 2014 Fine-tuning dual emission and aggregation-induced emission switching in NPI–BODIPY dyads Chem. Eur. J. 20 9052
Meares A, Satraitis A and Ptaszek M 2017 BODIPY-bacteriochlorin energy transfer arrays: toward near-IR emitters with broadly tunable, multiple absorption bands J. Org. Chem. 82 13068
Shao S, Thomas M B, Part K H, Mahaffey Z, Kim D and DSouza F 2018 Sequential energy transfer followed by electron transfer in a BODIPY-bisstyrylBODIPY bound to C60 triad via a two-point binding strategy Chem. Commun. 54 54
Wanger R W and Lindsey J S 1996 Boron-dipyrromethene dyes for incorporation in synthetic multi-pigment light-harvesting arrays Pure Appl. Chem. 68 1373
Wang J, Xu Z, Zhao Y, Qiao W and Li Z 2007 Synthesis and characterization of novel fluorescent surfactants Dyes Pigm. 74 103
Li X, Qian Q and Jiang W 2023 Photo-induced fluorochromism of a star-shaped photochromic dye with 2,4-dimethylthiazole attaching to triangle terthiophene J. Fluoresc. https://doi.org/10.1007/s10895-023-03196-1
Li X, Cai Q, Zhang J, Kim H and Son Y A 2020 An “electron lock” toward the photochromic activity of phenylacetylene appended bisthienylethene Mol. Cryst. Liq. Cryst. 706 141
Manna B, Ghosh R and Palit D K 2016 Ultrafast energy transfer process in doped-anthracene nanoaggregates is controlled by exciton diffusion: multiple doping leads to efficient white light emission J. Phys. Chem. C 120 7299
Lantzsch G, Binder H and Heerklotz H 1994 Surface area per molecule in lipid/C12En membranes as seen by fluorescence resonance energy transfer J. Fluoresc. 4 339
Maus M, De R, Lor M, Weil T, Mitra S, Wiesler U M, et al. 2001 Intramolecular energy hopping and energy trapping in polyphenylene dendrimers with multiple peryleneimide donor chromophores and a terryleneimide acceptor trap chromophore J. Am. Chem. Soc. 123 7668
Ventura J, Uriel C, Gómez AM, Avellanal-Zaballa E, Baňuelos J, Rebollar E, et al. 2023 4,4′-Dicyano- versus 4,4′-difluoro-BODIPYs in chemoselective postfunctionalization reactions: synthetic advantages and applications Org. Lett. 25 2588
Zheng X, Liu X, Liu L, Li X, Jiang S, Niu C, et al. 2022 Multi‐stimuli‐induced mechanical bending and reversible fluorescence switching in a single organic crystal Angew. Chem., Int. Ed. 61 e202113073
Xie P, Zhou Y. Li X, Liu X, Liu L, Cao Z, et al. 2023 Strong dual-state emission of unsymmetrical and symmetrical thiazolothiazole-bridged imidazolium salts Chin. Chem. Lett. 34 107582
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 21772034). We also thank the financial support from Henan Key Laboratory of Organic Functional Molecules and Drug Innovation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
About this article
Cite this article
Li, X., Yao, C. & Jiang, W. Emission and energy transfer investigation of non-conjugated total carbon configuration between BODIPY and naphthalimide. J Chem Sci 135, 65 (2023). https://doi.org/10.1007/s12039-023-02181-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-023-02181-2