Abstract
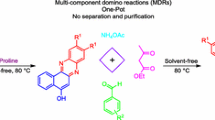
A series of tetrahydrobenzo[b]pyrans derivatives were synthesized with substituted pyrazole carbaldehydes, malononitrile and dimedone by ecofriendly L-proline catalyst in aqueous ethanol. The synthesized compounds were characterized using FTIR, 1H NMR, 13C NMR and Mass spectral techniques. This method holds the advantages of one-pot multicomponent, simple synthetic route, mild reaction conditions, high yield, use of less toxic chemicals and use of eco-friendly catalyst. We also report the study of the synthetic protocol by green chemistry metrics indicates a green relevance. Also, synthesized compounds were screened for their anti-inflammatory and antioxidant activity. Most of the tetrahydrobenzo[b]pyrans derivatives exhibit excellent activity.
Graphical abstract

A series of tetrahydrobenzo[b]pyrans derivatives were synthesized with substituted pyrazole carbaldehydes, malononitrile and dimedone using eco-friendly L-proline as a catalyst in aqueous ethanol. We also report the examination of the synthetic protocol by green chemistry metrics suggests that synthetic protocol has green relevance. Anti-inflammatory activity and antioxidant activity for tetrahydrobenzo[b]pyran derivatives are performed. Some of the synthesized compounds showed excellent anti-inflammatory as well as antioxidant activity.




Similar content being viewed by others
References
Thumar N J and Patel M P 2009 Synthesis and in vitro antimicrobial evaluation of 4H-pyrazolopyran-benzopyran and naphthopyran derivatives of 1H-pyrazole ARKIVOC (xiii). 363
Isaac GS, Eugenia M and Raquel PH 2015 Enantioselective organocatalyzed synthesis of 2-amino-3-cyano-4h-chromene derivatives Symmetry 7 1519
Youcef M and Erik DC 2010 Twenty-six years of anti-HIV drug discovery: Where do we stand and where do we go? J. Med. Chem. 53 521
William K, John D, Songchun J, Hong Z, Yan W, Jianghong Z, et al. 2004 Discovery of 4-Aryl-4H-chromenes as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay J. Med. Chem. 47 6299
Mohamed AR, Naima K, Saida B and Abdelmadjid D 2019 [DMImd-DMP]: A highly efficient and reusable catalyst for the synthesis of 4H-benzo[b]pyran derivatives Heterocycl. Commun. 25 167
Zahed K and Baharak P 2012 A facile synthesis of new 2-amino-4H-pyran-3-carbonitriles by a one-pot reaction of α,α-bis(arylidene) cycloalkanones and malononitrile in the presence of K2CO3 Sci. World J. 1
Khalid K, Smaail R, Youssef R, Jamal T, Yahia N M, Faiz A A and M’hammed A 2018 Synthesis and pharmacological activities of pyrazole derivatives: A review Molecules 23 134
Guan ZY, Xiao-Fei S, Pi-Le C, Xiao-Dan Y, Jia-Kai Z, Ying-Qian L, et al. 2018 Facile three-component synthesis, insecticidal and antifungal evaluation of novel dihydropyridine derivatives Molecules 23 2422
Magdy E A Z, Hanan A S, Ola A H and Aymn E R 2006 Pyrazolopyranopyrimidines as a class of anti-inflammatory agents Zeitsch. Naturfor. C 61 1
Florian S, Stefan TAB, Grygoriy YR, Kurt P and Herbert M 2007 Electrophilicity of 5-benzylidene-1,3-dimethylbarbituric and -thiobarbituric acids J. Org. Chem. 72 9170
Ahmad S, Sima S and Abbas R 2007 One-pot, three-component condensation reaction in water: An efficient and improved procedure for the synthesis of pyran annulated heterocyclic systems Synth. Commun. 37 491
Ipsita D, Kumar BSD and Pulak JB 2003 A novel three-component one-pot synthesis of pyrano[2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines using microwave heating in the solid state Tetrahedron Lett. 44 8307
Mohammad S and Hassan S 2008 High surface area MgO as a highly effective heterogeneous base catalyst for three-component synthesis of tetrahydrobenzopyran and 3,4-dihydropyrano[c]chromene derivatives in aqueous media Catal. Lett. 126 275
Goutam B and Bubun B 2014 Facile and one-pot access to diverse and densely functionalized 2-amino-3-cyano-4H-pyrans and pyran-annulated heterocyclic scaffolds via an eco-friendly multicomponent reaction at room temperature using urea as a novel organo-catalyst ACS Sustain. Chem. Eng. 2 411
Naglaa M, Abd E and Rita M B 2014 Eco-friendly solvent-free synthesis of tetrahydrobenzo[b]pyran World Appl. Sci. J. 31 1
Sougata S, Matiur R, Anupam R, Adinath M and Alakananda H 2014 Microwave-assisted three-component ‘‘catalyst and solvent-free’’ green protocol: A highly efficient and clean one-pot synthesis of tetrahydrobenzo[b]pyrans Org. Chem. Int. 1
Mithu S and Amarta K P 2012 Palladium (0) nanoparticles: A novel and reusable catalyst for the synthesis of various pyran derivatives Adv. Nano 1 61
Ali A M, Mohammad R A and Armin H 2017 Synthesis of tetrahydrobenzo[b]pyran under catalysis of NH4Al(SO4)2·12H2O (Alum) Arab. J. Chem. 10
Liangliang H, Xiaoyun H and Zhongqiang Z 2016 Diammonium hydrogen phosphate as a recyclable catalyst for the rapid and green synthesis of 2-amino-1,4,5,6 tetrahydropyrano[3,2-c]-quinolin-5-one derivatives Polycycl. Arom. Comp. 37 1
Huanan H, Fangli Q, Anguo Y, Jianguo Y and Haiping M 2014 An environmentally benign protocol for aqueous synthesis of tetrahydrobenzo[b]pyrans catalyzed by cost-effective ionic liquid Int. J. Mol. Sci. 15 6897
Ghodsi M Z, Alireza A, Alireza B and Zeinab A 2011 An efficient synthesis of tetrahydrobenzo[b]pyran derivatives using sulfonic acid functionalized silica as an efficient catalyst E-J Chem. 8 293
Mousavi M R, Maghsoodlou M T, Noori F and Hazeri N 2015 A facile and efficient synthesis of tetrahydrobenzo[b]pyrans using sucrose as green, inexpensive, natural and biodegradable catalyst Org. Chem. Res. 1 66
Sayyedeh S P, Sayyed M H and Mehdi S 2015 Fructose-catalyzed synthesis of tetrahydrobenzo[b]pyranderivatives: Investigation of kinetics and mechanism Chin. J. Catal. 36 757
Pandit V U, Arbuj S S, Pandit Y, Naik S, Rane S, Mulik U, et al. 2015 Solar light driven dye degradation using novel organo–inorganic (6,13-pentacenequinone/TiO2) nanocomposite RSC Adv. 5 10326
Pandit V, Arbuj S, Mulik U and Kale B 2014 Novel functionality of organic 6,13-pentacenequinone as a photocatalyst for hydrogen production under solar light Environ. Sci. Technol. 48 4178
Pandit V, Arbuj S, Hawaldar R, Kshirsagar P, Ambekar J, Mulik U, et al. 2015 Hierarchical CdS nanostructure by Lawesson’s reagent and its enhanced photocatalytic hydrogen production RSC Adv. 5 13715
Pandit V, Arbuj S, Hawaldar R, Kshirsagar P, Mulik U, Gosavi S, et al. 2015 In situ preparation of a novel organo-inorganic 6,13-pentacenequinone–TiO2 coupled semiconductor nanosystem: A new visible light active photocatalyst for hydrogen generation J. Mater. Chem. A 5 4338
Kumbhar D, Pandit V, Deshmukh S, Ambekar J, Arbuj S and Rane S 2015 Synthesis of hierarchical ZnO nanostructure and its photocatalytic performance study J. Nano. Nanoman. 5 227
Nevase K, Arbuj S, Pandit V, Ambekar J and Rane S 2015 Synthesis, characterization and photocatalytic activity of tungsten oxide nanostructures J. Nano. Nanoman. 5 221
Pandit S, Bhalerao S, Aher U, Adhav G and Pandit V 2011 Amberlyst A-15: Reusable catalyst for the synthesis of 2, 4, 5-trisubstituted and 1, 2, 4, 5-tetrasubstituted-1H-imidazoles under MW irradiation J. Chem. Sci. 4 421
Pandit S, Shaikh R and Pandit V 2009 Synthesis of 5-unsubstituted-3, 4-dihydropyridine-2-(1h)-ones using nbs as a catalyst under solvent free conditions Rasayan J. Org. Chem. 2 907
Somwanshi A S, Pandit S S, Ghogare R D, Pandit V and Gholap AD 2018 Guanidine carbonate: Efficient catalyst for knoevenagel condensation of aryl aldehydes with Meldrum’s acid Int J. Chem. Phys. Sci. 7 92
Jawale V, Gugale G, Chaskar M, Pandit S, Pawar R, Suryawanshi S, et al. 2021 Two-and three-dimensional zinc oxide nanostructures and its photocatalytic dye degradation performance study J. Mater. Res. 36 1573
Dhevalapally R and Mamillapalli K 2008 Direct amino acid-catalyzed cascadebiomimetic reductive alkylations:application to the asymmetric synthesis of Hajos-Parrish ketone analogues Org. Biomol. Chem. 6 4176
Dhevalapally R and Mamillapalli K 2010 Direct catalytic asymmetric synthesis of highly functionalized tetronicacids/tetrahydroisobenzofuran-1,5-diones via combination of cascadethree-component reductive alkylations and Michael-aldol reactions Org. Biomol. Chem. 8 2859
Dhevalapally R and Mamillapalli K 2007 Organocatalytic sequential one-pot double cascade asymmetric synthesis of Wieland–Miescher Ketone analogues from a knoevenagel/hydrogenation/robinson annulation sequence: Scope and applications of organocatalytic biomimetic reductions J. Org. Chem. 72 5056
Dhevalapally R, Mamillapalli K and Reddy Y 2008 Development of Pharmaceutical Drugs, Drug Intermediates and Ingredients by Using Direct Organo-Click Reactions Eur. J. Org. Chem. 975
Dhevalapally R and Reddy Y 2010 A general approach to chiral building blocks via direct amino acid-catalyzed cascade three-component reductive alkylations: Formal total synthesis of HIV-1 protease inhibitors, antibiotic agglomerins, brefeldin A, and (R)-γ-hexanolide J. Org. Chem. 75 74
Hossein A O, Majid M H, Narges K and Mojgan E Z 2011 Caro's acid-silica gel: An efficientand versatile catalyst for the one-pot synthesis of tetrahydrobenzo[b]pyran derivatives Synth. Commun. 41 436
Anastas P T and Warner J 1998 Green chemistry theory and practice (Oxford University Press: Oxford, UK)
Martyn P, Michael F J, Trevor R F and Paul T A 2002 Green chemistry: Science and politics of change Science 297 5582
Alan D C, David J C C, David N M and Virginia L C 2001 So you think your process is green, how do you know? Using principles of sustainability to determine what is green-a corporate perspective Green Chem. 3 1
Claudio O K, Laura I R and Rita H R 2009 The development of an environmentally benign sulfide oxidation procedure and its assessment by green chemistry metrics Green Chem. 11 223
Jamale D K, Vibhute S S, Undare S S, Valekar N J, Patil K T, Warekar P P, et al. 2018 Unexpected formation of 4,5-dihydro-1H-pyrazolo[3,4-b]pyridine derivatives as a potent antitubercular agent and its evaluation by green chemistry metrics Synth. Commun. 48 21
Kate P R, Gaikwad S H, Lokhande T N, Shaikh A B, Sonawane B, Choudhari P and Bachute M T 2018 Synthesis of schiff base as DNA gyrase B inhibitor, antibacterial, anti-Inflammatory and antioxidant agents Rasayan J. Chem. 11 1441
Sirsat D, Kate P R and Bachute M T 2019 Synthesis and biological evaluation of novel thiazole-pyrazole integrated chalcones as antioxidant and anti-inflammatory agents Asian J. Pharm. Clin. Res. 12 311
Acknowledgements
Author(s) are thankful to the Principal of Karmaveer Bhaurao Patil Mahavidyalaya, Pandharpur, Shri. Shivaji Mahavidyalaya, Barshi and The Poona Gujarati Kelwani Mandal’s Haribhai V. Desai College, Pune (Dr. Ganesh Raut) for providing essential facilities. Author (s) are also thankful to the Department of instrumentation Punyashlok Ahilyadevi Holkar Solapur University, Solapur for providing spectral characterization.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
KATE, P., PANDIT, V., JAWALE, V. et al. L-Proline catalyzed one-pot three-component synthesis and evaluation for biological activities of tetrahydrobenzo[b]pyran: evaluation by green chemistry metrics. J Chem Sci 134, 4 (2022). https://doi.org/10.1007/s12039-021-01990-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-021-01990-7