Abstract
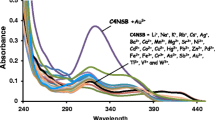
A new anthraquinone appended oxacalix[4]arene (DAQOC) has been synthesized and characterized by different 1H NMR, IR, ESI-MS and 13C NMR spectroscopic techniques. This compound was explored for its sensing abilities towards various anions. DAQOC showed selective anion sensing behaviour towards F− ions which was supported by absorption as well as emission studies. Among other anions, only in the presence of F− ions, a quenching in the fluorescence emission of over 79% was observed due to changes in the intermolecular charge transfer (ICT) process. DAQOC exhibited high selectivity and good sensitivity toward F− ions in the presence of competing ions and the detection limit was found to be 1.23 µM. 1H NMR titration displays that the peak corresponding to –NH protons (at 12.91 ppm) disappears upon interaction with F−, suggesting that the sensing mechanism follows the deprotonation route. The geometrical features of F− bound oxacalixarene species were modelled by the Density Functional Theory (DFT) and NCIPlot calculations. The findings suggested that the appended substituents including nitro groups and anthraquinone can make the calix[4]arene ring electron deficient and thereby more susceptible for F− ions. Moreover, this present chemosensor has been applied for recognition of F− ions from waste water samples which is of direct practical relevance.
Graphic abstract
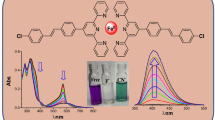
A new anthraquinone appended oxacalix[4]arene has been explored for its sensing abilities towards F− by absorption and emission studies. An intermolecular charge transfer mechanism has been attributed due to the deprotonation of N–H groups in DAQOC. DAQOC-F− complex is modelled by the Density Functional Theory and NCIPlot calculations.










Similar content being viewed by others
References
Hein R, Beer P D and Davis J J 2020 Electrochemical anion sensing: supramolecular approaches Chem. Rev. 120 1888
Pollick H 2018 The role of fluoride in the prevention of tooth decay Pediatr. Clin. 65 923
Pereira A G, Chiba F Y, Mattera, M S d L C, Pereira R F, Nunes R d C A, Tsosura T V S, Okamoto R and Sumida D H 2017 Effects of fluoride on insulin signaling and bone metabolism in ovariectomized rats J. Trace. Elem. Med. Bio. 39 140
Kanduti D, Sterbenk P and Artnik B 2016 Fluoride: a review of use and effects on health Mater. Sociomed. 28 133
Black R M and Read R W 2007 Environmental and biomedical sample analysis in support of allegations of use of chemical warfare agents Toxin Rev. 26 275
Kingery A F and Allen H E 1995 The environmental fate of organophosphorus nerve agents: a review Toxicol. Environ. Chem. 47 155
Busschaert N, Caltagirone C, Van Rossom W and Gale P A 2015 Applications of supramolecular anion recognition Chem. Rev. 115 8038
Kumar R, Sharma A, Singh H, Suating P, Kim H S, Sunwoo K, Shim I, Gibb B C and Kim J S 2019 Revisiting fluorescent calixarenes: from molecular sensors to smart materials Chem. Rev. 119 9657
Mehta V, Panchal M, Modi K, Kongor A, Panchal U and Jain V K 2015 The chemistry of nascent oxacalix [n] hetarene (n ≥ 4): a review Curr. Org. Chem. 19 1077
Panchal M, Kongor A, Athar M, Modi K, Patel C, Dey, S, Vora M, Bhadresha K, Rawal R, Jha P C and Jain V K 2020 Structural motifs of oxacalix [4] arene for molecular recognition of nitroaromatic explosives: experimental and computational investigations of host-guest complexes J. Mol. Liq. 112809
Wu J R and Yang Y W 2019 New opportunities in synthetic macrocyclic arenes Chem. Comm. 55 1533
Ma J X, Fang X, Xue M and Yang Y 2019 Synthesis, structure, and anion binding of functional oxacalix [4] arenes Org. Biomol. Chem. 17 5075
Van Rossom W, Caers J, Robeyns K, Van Meervelt L, Maes W and Dehaen W 2012 (Thio) ureido anion receptors based on a 1, 3-alternate oxacalix [2] arene [2] pyrimidine scaffold J. Org. Chem. 77 2791
Hou B Y, Wang D X, Yang H B, Zheng Q Y and Wang M X 2007 Synthesis and structure of upper-rim 1, 3-alternate tetraoxacalix [2] arene [2] triazine azacrowns and change of cavity in response to fluoride anion J. Org. Chem. 72 5218
An L, Wang J W, Wang C, Zhou S S, Sun J and Yan C G 2018 2, 3-Ethylene-bridged dihomooxacalix [4] arenes: synthesis, X-ray crystal structures and highly selective binding properties with anions New J. Chem. 42 10689
Liu W, Wang Q Q, Huang Z T and Wang D X 2014 Design, structure and anion recognition of larger-rim functionalized oxacalix [2] arene [2] triazine hosts Tetrahedron Lett. 55 3172
Augusto A S, Miranda A S, Ascenso J R, Miranda M Q, Félix V, Brancatelli G, Hickey N, Geremia S and Marcos P M 2018 Anion recognition by partial cone dihomooxacalix[4] arene-based receptors bearing urea groups: remarkable affinity for benzoate ion Eur. J. Org. Chem. 2018 5657
Panchal M K, Athar M, Jha P C, Kongor A, Mehta V and Jain V K 2017 Quinoline appended oxacalixarene as turn-off fluorescent probe for the selective and sensitive determination of Cu2+ ions: a combined experimental and DFT study J. Lumin. 192 256
Katz J L, Feldman M B and Conry R R 2005 Synthesis of functionalized oxacalix[4] arenes Org. Lett. 7 91
Mehta V, Athar M, Jha P C, Panchal M K, Modi K and Jain V K 2016 Efficiently functionalized oxacalix[4] arenes: synthesis, characterization and exploration of their biological profile as novel HDAC inhibitors Bioorg. Med. Chem. Lett. 26 1005
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricat, M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg J L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A, Peralta J E, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J C, Iyengar S S, Tomasi J, Cossi M, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, Dapprich S, Daniels A D, Farkas, Foresman J B, Ortiz J V, Cioslowski J and Fox D J 2009 Gaussian 09, Revision B.01. Wallingford CT
Grimme S 2006 Semiempirical GGA-type density functional constructed with a long-range dispersion correction J. Comput. Chem. 27 1787
Athar M, Lone M Y and Jha P C 2017 Investigation of structural and conformational equilibrium of Oxacalix [4] arene: a density functional theory approach J. Mol. Liq. 237 473
Boys S F and Bernardi F D 1970 The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors Mol. Phys. 19 553
Boys S and Bernardi F 2002 The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors Mol. Phys. 100 65
Contreras-García J, Johnson E R, Keinan S, Chaudret R, Piquemal J P, Beratan D N, Yang W 2011 NCIPLOT: a program for plotting noncovalent interaction regions Chem. Theory. Comput. 7 625
Batista R M, Costa S P and Raposo M M M 2013 Naphthyl-imidazo-anthraquinones as novel colorimetric and fluorimetric chemosensors for ion sensing J. Photochem. Photobiol. A 259 33
Jones J E, Kariuki B M, Ward B D and Pope S J 2011 Amino-anthraquinone chromophores functionalised with 3-picolyl units: structures, luminescence, DFT and their coordination chemistry with cationic Re (I) di-imine complexes Dalton Trans. 40 3498
Devaraj S, Saravanakumar D and Kandaswamy M 2007 Dual chemosensing properties of new anthraquinone-based receptors toward fluoride ions Tetrahedron Lett. 48 3077
Langdon-Jones E E and Pope S J 2014 The coordination chemistry of substituted anthraquinones: developments and applications Coord. Chem. Rev. 269 32
Hoten M, Kojima Y and Ito T 1992 Halochromism of acrylic fibres dyed with some disperse dyes J. Soc. Dyers Colour. 108 21
Chen X, Leng T, Wang C, Shen Y and Zhu W 2017 A highly selective naked-eye and fluorescent probe for fluoride ion based on 1,8-naphalimide and benzothizazole Dyes Pigm. 141 299
Gunnlaugsson T, Davis A P, O’Brien J E and Glynn M 2005 Synthesis and photophysical evaluation of charge neutral thiourea or urea based fluorescent PET sensors for bis-carboxylates and pyrophosphate Org. Biomol. Chem. 3 48
Bobe S R, Bhosale S V, Jones L, Puyad A L, Raynor A M and Bhosale S V 2015 A near-infrared fluoride sensor based on a substituted naphthalenediimide–anthraquinone conjugate Tetrahedron Lett. 56 4762
Poulsen J R and Birks J W 1989 Photoreduction fluorescence detection of quinones in high-performance liquid chromatography Anal. Chem. 61 2267
Sood D, Kumar N, Singh A, Tomar V, Dass S K and Chandra R 2019 Deciphering the binding mechanism of noscapine with lysozyme: biophysical and chemoinformatic approaches ACS Omega 4 16233
Inoue H, Hida M, Nakashima N and Yoshihara K 1982 Picosecond fluorescence lifetimes of anthraquinone derivatives. Radiationless deactivation via intra-and intermolecular hydrogen bonds Open J. Phys. Chem. 86 3184
Jung H S, Kim H J, Vicens J and Kim J S 2009 A new fluorescent chemosensor for F− based on inhibition of excited-state intramolecular proton transfer Tetrahedron Lett. 50 983
Maity D, Chakraborty A, Gunupuru R and Paul P 2011 Calix[4] arene based molecular sensors with pyrene as fluoregenic unit: effect of solvent in ion selectivity and colorimetric detection of fluoride Inorg. Chim. Acta 372 126
Chawla H M, Shrivastava R and Sahu S N 2008 A new class of functionalized calix [4] arenes as neutral receptors for colorimetric detection of fluoride ions New J. Chem. 32 1999
Qureshi N, Yufit D S, Steed K M, Howard J A and Steed J W 2014 Hydrogen bonding effects in anion binding calixarenes CrystEngComm 16 8413
Wang D X and Wang M X 2013. Anion-π interactions: generality, binding strength, and structure J. Am. Chem. Soc. 135 892
Acknowledgement
The authors thank the financial assistance provided by DST-SERB, New Delhi through the project scheme SERB REF. No EMR/2016/001958. Manoj Vora gratefully acknowledges the financial support received from UGC in the form of Rajiv Gandhi National Fellowship, RGNF-JRF (RGNF-2017-18-SC-GUJ-47048). V. K. Jain would like to acknowledge UGC-Mid Career Award for financial assistance (19-213/2018-BSR). Anita Kongor gratefully acknowledges the financial assistance provided by the Council of Scientific & Industrial Research (CSIR), New Delhi for Research Associateship Fellowship (File No. 09/070 (0073) 2020 EMR-I). Manthan K. Panchal gratefully acknowledges Human Resource Development Group - Council of Scientific & Industrial Research (CSIR), New Delhi for Research Associateship Fellowship (File No. 09/70 (0064) 2K19 EMR-I). The authors also acknowledge the Central Salt & Marine Chemicals Research Institute (Bhavnagar), Oxygen Healthcare-Ahmedabad (O2h), Sophisticated Analytical Instrument Facility (Panjab University) and Gujarat Forensic Sciences University (Gandhinagar), for providing instrumental facilities and UGC Infonet & Information and Library Network (INFLIBNET) (Ahmedabad) for e-journals.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
VORA, M., KONGOR, A., PANCHAL, M. et al. A highly selective anthraquinone appended oxacalixarene receptor for fluorescent ICT sensing of F− ions: an experimental and computational study. J Chem Sci 132, 156 (2020). https://doi.org/10.1007/s12039-020-01862-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-020-01862-6