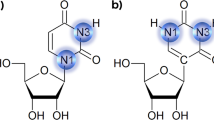
Abstract
Urea-assisted denaturation of protein and RNA has been shown to be a valuable tool to study their stabilities and folding phenomena. It has been shown that stacking interactions between nucleobases and urea are one of the driving forces of denaturation. In this study, the ability of urea to form unconventional stacking interactions with RNA bases is investigated by performing high-level quantum calculations (RI-MP2/aug-cc-pVDZ level) on a few thousands of model systems. Four systems were considered based on the RNA nucleobases (GUA, ADE, CYT, and URA) for the investigation. For each system, a set of models were designed to study the role of hetero-atoms/groups of the nucleobases on stacking interactions with urea moiety with respect to every possible pair. Several plane-parallel complexes were generated with urea on top of aromatic systems to exhaustively study all possible factors for urea-nucleobases stacking interactions. Energy decomposition analysis (EDA), atoms in molecules (AIM) and natural bond orbital (NBO) analysis were performed to gain better insights on non-covalent stacking interactions. Dispersion component was found to be heavily stabilizing, while the \(\hbox {E}_\mathrm{HF}\) was found to be repulsive for all the four systems indicating lack of hydrogen bonding (HB) type interactions and presence of dispersion type interactions. Amide and carbonyl groups of urea molecule were found to play a major role in favourable stacking interactions. We demonstrate that along with functional groups present on the nucleobases, the orientation of urea molecules plays a vital role in stabilizing the urea-nucleobase non-covalent interactions. The proposed study quantifies and provides a comprehensive theoretical description of urea nucleobase unconventional stacking interactions which helps to unravel urea driven RNA unfolding mechanism.
Graphical Abstract
SYNOPSIS Position and orientation effects on the stacking interactions between the urea and nucleobases, the driving force in urea-induced RNA denaturation, were studied using quantum mechanical calculations. Dispersion effects, functional groups of the bases and orientation of urea molecules are the key contributing factors affecting these interactions.










Similar content being viewed by others
References
Mercer T R, Dinger M E and Mattick J S 2009 Long non-coding RNAs: insights into functions Nat. Rev. Genet. 10 155
Breaker R R, Gesteland R F, Cech T R and Atkins J F 2006 The RNA world (New York: Cold Spring Harbor Laboratory Press)
Blower M D 2013 Molecular insights into intracellular RNA localization Int. Rev. Cell Mol. Biol. 302 1
Burg M B and Ferraris J D 2008 Intracellular organic osmolytes: function and regulation J. Biol. Chem. 283 7309
Yancey P H 2005 Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses J. Exp. Biol. 208 2819
Santoro M M, Liu Y, Khan S M A, Hou L X and Bolen D W 1992 Increased thermal stability of proteins in the presence of naturally occurring osmolytes Biochemistry (Mosc.) 31 5278
Wang A and Bolen D W 1997 A naturally occurring protective system in urea-rich cells: Mechanism of osmolyte protection of proteins against urea denaturation Biochemistry (Mosc.) 36 9101
Schein C H 1990 Solubility as a function of protein structure and solvent components Nat. Biotechnol. 8 308
Zhou R, Li J, Hua L, Yang Z and Berne B J 2011 Comment on “urea-mediated protein denaturation: A consensus view” J. Phys. Chem. B 115 1323
Li W, Zhou R and Mu Y 2012 Salting effects on protein components in aqueous NaCl and urea solutions: toward understanding of urea-induced protein denaturation J. Phys. Chem. B 116 1446
Wallqvist A, Covell D G and Thirumalai D 1998 Hydrophobic interactions in aqueous urea solutions with implications for the mechanism of protein denaturation J. Am. Chem. Soc. 120 427
Guinn E J, Pegram L M, Capp M W, Pollock M N and Record M T 2011 Quantifying why urea is a protein denaturant, whereas glycine betaine is a protein stabilizer Proc. Natl. Acad. Sci. 108 16932
Hua L, Zhou R, Thirumalai D and Berne B J 2008 Urea denaturation by stronger dispersion interactions with proteins than water implies a 2-stage unfolding Proc. Natl. Acad. Sci. 105 16928
Lambert D and Draper D E 2012 Denaturation of RNA secondary and tertiary structure by urea: simple unfolded state models and free energy parameters account for measured m-values Biochemistry (Mosc.) 51 9014
Priyakumar U D, Hyeon C, Thirumalai D and MacKerell Jr A D 2009 Urea destabilizes RNA by forming stacking interactions and multiple hydrogen bonds with nucleic acid bases J. Am. Chem. Soc. 131 17759
Shelton V M, Sosnick T R and Pan T 1999 Applicability of urea in the thermodynamic analysis of secondary and tertiary RNA folding Biochemistry (Mosc.) 38 16831
Canchi D R, Paschek D and García A E 2010 Equilibrium study of protein denaturation by urea J. Am. Chem. Soc. 132 2338
Stumpe M C and Grubmüller H 2007 Interaction of urea with amino acids: implications for urea-induced protein denaturation J. Am. Chem. Soc. 129 16126
Guinn E J, Schwinefus J J, Cha H K, McDevitt J L, Merker W E, Ritzer R, Muth G W, Engelsgjerd S W, Mangold K E and Thompson P J 2013 Quantifying functional group interactions that determine urea effects on nucleic acid helix formation J. Am. Chem. Soc. 135 5828
Ma L, Pegram L, Record Jr M T and Cui Q 2010 Preferential interactions between small solutes and the protein backbone: A computational analysis Biochemistry (Mosc.) 49 1954
Auton M and Bolen D W 2005 Predicting the energetics of osmolyte-induced protein folding/unfolding Proc. Natl. Acad. Sci. U. S. A. 102 15065
Baldwin R L 1975 Intermediates in protein folding reactions and the mechanism of protein folding Annu. Rev. Biochem. 44 453
Makhatadze G I and Privalov P L 1992 Protein interactions with urea and guanidinium chloride: a calorimetric study J. Mol. Biol. 226 491
Goyal S, Chattopadhyay A, Kasavajhala K and Priyakumar U D 2017 Role of Urea–Aromatic Stacking Interactions in Stabilizing the Aromatic Residues of the Protein in Urea-Induced Denatured State J. Am. Chem. Soc. 139 14931
Schneck E, Horinek D and Netz R R 2013 Insight into the molecular mechanisms of protein stabilizing osmolytes from global force-field variations J. Phys. Chem. B 117 8310
Bennion B J and Daggett V 2003 The molecular basis for the chemical denaturation of proteins by urea Proc. Natl. Acad. Sci. 100 5142
Levy Y and Onuchic J N 2006 Water mediation in protein folding and molecular recognition Annu. Rev. Biophys. Biomol. Struct. 35 389
Vanzi F, Madan B and Sharp K 1998 Effect of the protein denaturants urea and guanidinium on water structure: A structural and thermodynamic study J. Am. Chem. Soc. 120 10748
Berteotti A, Barducci A and Parrinello M 2011 Effect of Urea on the \(\upbeta \)-Hairpin Conformational Ensemble and Protein Denaturation Mechanism J. Am. Chem. Soc. 133 17200
Stumpe M C and Grubmüller H 2007 Aqueous urea solutions: structure, energetics, and urea aggregation J. Phys. Chem. B 111 6220
Guinn E J, Pegram L M, Capp M W, Pollock M N and Record M T 2011 Quantifying why urea is a protein denaturant, whereas glycine betaine is a protein stabilizer Proc. Natl. Acad. Sci.
Liu C, Wang T, Bai Y and Wang J 2017 Electrostatic forces govern the binding mechanism of intrinsically disordered histone chaperones PLOS ONE 12 e0178405
Rocco A G, Mollica L, Ricchiuto P, Baptista A M, Gianazza E and Eberini I 2008 Characterization of the protein unfolding processes induced by urea and temperature Biophys. J. 94 2241
Sánchez I E and Kiefhaber T 2003 Evidence for sequential barriers and obligatory intermediates in apparent two-state protein folding J. Mol. Biol. 325 367
Streicher W W and Makhatadze G I 2007 Unfolding thermodynamics of Trp-cage, a 20 residue miniprotein, studied by differential scanning calorimetry and circular dichroism spectroscopy Biochemistry (Mosc.) 46 2876
England J L and Haran G 2011 Role of solvation effects in protein denaturation: from thermodynamics to single molecules and back Annu. Rev. Phys. Chem. 62 257
Miner J C and García A E 2017 Equilibrium Denaturation and Preferential Interactions of an RNA Tetraloop with Urea J. Phys. Chem. B 121 3734
Herschlag D 1995 RNA chaperones and the RNA folding problem J. Biol. Chem. 270 20871
Kiger A A, Baum B, Jones S, Jones M R, Coulson A, Echeverri C and Perrimon N 2003 A functional genomic analysis of cell morphology using RNA interference J. Biol. 2 27
Mercer T R and Mattick J S 2013 Structure and function of long noncoding RNAs in epigenetic regulation Nat. Struct. Mol. Biol. 20 300
McKay D B 1996 Structure and function of the hammerhead ribozyme: an unfinished story RNA 2 395
Wada A and Suyama A 1986 Local stability of DNA and RNA secondary structure and its relation to biological functions Prog. Biophys. Mol. Biol. 47 113
Lambert D and Draper D E 2007 Effects of osmolytes on RNA secondary and tertiary structure stabilities and RNA-Mg2+ interactions J. Mol. Biol. 370 993
Pincus D L, Hyeon C and Thirumalai D 2008 Effects of trimethylamine N-oxide TMAO and crowding agents on the stability of RNA hairpins J. Am. Chem. Soc. 130 7364
Yoon J, Thirumalai D and Hyeon C 2013 Urea-induced denaturation of preQ1-riboswitch J. Am. Chem. Soc. 135 12112
Kasavajhala K, Bikkina S, Patil I, MacKerell Jr A D and Priyakumar U D 2015 Dispersion interactions between urea and nucleobases contribute to the destabilization of RNA by urea in aqueous solution J. Phys. Chem. B 119 3755
Salonen L M, Ellermann M and Diederich F 2011 Aromatic rings in chemical and biological recognition: energetics and structures Angew. Chem. Int. Ed. 50 4808
Schottel B L, Chifotides H T and Dunbar K R 2008 Anion-\(\uppi \) interactions Chem. Soc. Rev. 37 68
Gallivan J P and Dougherty D A 1999 Cation-\(\uppi \) interactions in structural biology Proc. Natl. Acad. Sci. 96 9459
Hunter C A, Lawson K R, Perkins J and Urch C J 2001 Aromatic interactions J. Chem. Soc. Perkin Trans. 2 651
Knowles R R and Jacobsen E N 2010 Attractive noncovalent interactions in asymmetric catalysis: links between enzymes and small molecule catalysts Proc. Natl. Acad. Sci. 201006402
Zürcher M and Diederich F 2008 Structure-based drug design: exploring the proper filling of apolar pockets at enzyme active sites J. Org. Chem. 73 4345
Schneider H-J 2009 Binding mechanisms in supramolecular complexes Angew. Chem. Int. Ed. 48 3924
Weigend F, Häser M, Patzelt H and Ahlrichs R 1998 RI-MP2: optimized auxiliary basis sets and demonstration of efficiency Chem. Phys. Lett. 294 143
Schmidt M W, Baldridge K K, Boatz J A, Elbert S T, Gordon M S, Jensen J H, Koseki S, Matsunaga N, Nguyen K A and Su S 1993 General atomic and molecular electronic structure system J. Comput. Chem. 14 1347
Su P and Li H 2009 Energy decomposition analysis of covalent bonds and intermolecular interactions J. Chem. Phys. 131 014102
Reed A E, Curtiss L A and Weinhold F 1988 Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint Chem. Rev. 88 899
Todd A and Keith T K 2011 AIMALL Version 11.12. 19. Overland Park KS, USA: Gristmill Software
Kitaura K and Morokuma K 1976 A new energy decomposition scheme for molecular interactions within the Hartree-Fock approximation Int. J. Quantum Chem. 10 325
Glendening E D and Streitwieser A 1994 Natural energy decomposition analysis: An energy partitioning procedure for molecular interactions with application to weak hydrogen bonding, strong ionic, and moderate donor–acceptor interactions J. Chem. Phys. 100 2900
Lee E C, Kim D, Jurečka P, Tarakeshwar P, Hobza P and Kim K S 2007 Understanding of assembly phenomena by aromatic- aromatic interactions: benzene dimer and the substituted systems J. Phys. Chem. A 111 3446
Hunter C A and Sanders J K 1990 The nature of. pi.-. pi. interactions J. Am. Chem. Soc. 112 5525
Wheeler S E 2011 Local nature of substituent effects in stacking interactions J. Am. Chem. Soc. 133 10262
Wheeler S E 2012 Understanding substituent effects in noncovalent interactions involving aromatic rings Acc. Chem. Res. 46 1029
Wheeler S E and Houk K N 2008 Substituent Effects in the Benzene Dimer are Due to Direct Interactions of the Substituents with the Unsubstituted Benzene J. Am. Chem. Soc. 130 10854
Cheng X, Shkel I A, O’Connor K, Henrich J, Molzahn C, Lambert D and Record Jr M T 2017 Experimental Atom-by-Atom Dissection of Amide–Amide and Amide–Hydrocarbon Interactions in \(\text{H}_{2}\text{ O }\) J. Am. Chem. Soc. 139 9885
Blanco F, Kelly B, Sánchez-Sanz G, Trujillo C, Alkorta I, Elguero J and Rozas I 2013 Non-covalent interactions: complexes of guanidinium with DNA and RNA nucleobases J. Phys. Chem. B 117 11608
Blanco F, Kelly B, Alkorta I, Rozas I and Elguero J 2011 Cation–\(\uppi \) interactions: Complexes of guanidinium and simple aromatic systems Chem. Phys. Lett. 511 129
Acknowledgements
We thank DST SERB for financial assistance (Grant No. EMR/2016/007697).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alodia, N., Jaganade, T. & Priyakumar, U.D. Quantum mechanical investigation of the nature of nucleobase-urea stacking interaction, a crucial driving force in RNA unfolding in aqueous urea. J Chem Sci 130, 158 (2018). https://doi.org/10.1007/s12039-018-1563-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1563-8