Abstract
In this work, we have synthesised and crystallographically characterized a mononuclear iron(II) complex, [\(\hbox {Fe}(\hbox {phen})_{3}\)](\(\hbox {NO}_{3})_{2}\cdot 2\hbox {H}_{2}\hbox {O}\) (1) (phen = 1,10-phenanthroline). Single crystal X-ray diffraction (SXRD) analysis revealed that 1 crystallizes in monoclinic system with P \(1^{-}\) space group. The lattice water molecules in 1 form a water-nitrate cluster, \((\hbox {H}_{2}\hbox {O})_{2}^{\ldots }(\hbox {NO}_{3})_{2}\) through strong H-bonding interaction mediated via iron(II) complex in a unique binding motif and provide additional stability to the compound in the solid state. This iron(II) complex is able to catalyze the cleavage of aromatic C-C linkage of 2,5-dihydroxybenzoic acid (Gentisic acid, GA) in oxygen environment. The iron(II) complex in the presence of two equivalent of triethylamine \((\hbox {Et}_{3}\hbox {N})\) binds with GA stoichiometrically in acetonitrile medium at \(25\,{^{\circ }}\hbox {C}\). Observation of GA-to-iron LMCT optical bands at 521 and 609 nm supports in situ generated iron-GA adduct in solution. This in situ generated iron-GA adduct reacts with molecular oxygen at the rate, \(k_{\mathrm{obs}} = 6.58 \times 10^{-3}\,\mathrm{min}^{-1}\) in acetonitrile and affords exclusively 2-oxo-4-hydroxy-hepta-3,5-dienedioic acid. The incorporation of both the oxygen atoms of molecular oxygen in the bio-mimicking activity of gentisate-1,2-dioxygenase by this iron(II)-phenanthroline complex remain a rare example in scientific literature.
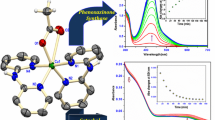
Graphical Abstract
A mononuclear iron(II) phenanthroline complex exhibits significant catalytic activity towards oxidative cleavage of 2,5-dihydroxy benzoic acid at a rate, \(\hbox {k}_{\mathrm{obs}}(\mathrm{min}^{-1}) = 6.58\times 10^{-3}\) which predominantly produced 2-oxo-4-hydroxy-hepta-3,5-dienedioic acid upon addition of 2,5-dihydroxybenzoic acid in the presence of molecular oxygen.







Similar content being viewed by others
References
Prince R H 1987 Comprehensive Coordination Chemistry G Wilkinson, J A McCleverty and R Gillard (Eds.) (Oxford: Pergamon) Vol. 5 p. 925; (b) Que L Jr 1989 Iron Carriers and Iron Proteins T M Loehr (Ed.) (New York: VCH) p. 467; (c) Biswas B, Mitra M, Adhikary J, Krishna G R, Bag P P, Reddy C M, Aliaga-Alcalde N, Chattopadhyay T, Das D and Ghosh R 2013 Synthesis, X-ray structural and magnetic characterizations, and epoxidation activity of a new bis(\(\upmu \)-acetato)(\(\upmu \)-alkoxo)dinuclear iron(III) complex Polyhedron 53 264; (d) Pal A, Biswas B, Mitra M, Purohit C S, Hazra S, Kumar G S and Ghosh R 2013 Synthesis, X-ray structure and DNA binding of a mononuclear iron(II) Schiff base complex J. Chem. Sci. 125 1161
(a) Que L Jr and Ho R Y N 1996 Dioxygen activation by enzymes with mononuclear non-heme iron active sites Chem. Rev. 96 2607; (b) Que L Jr, Lipscomb J D, Münck E and Wood J M 1977 Protocatechuate 3,4-dioxygenase: Inhibitor studies and mechanistic implications Biochim. Biophys. Acta 485 60; (c) Biswas B, Al-Hunaiti A, Räisänen M T, Ansalone S, Leskelä M, Repo T, Chen Y T, Tsai H L, Naik A D, Railliet A P, Garcia Y, Ghosh R and Kole N 2012 Efficient and selective oxidation of primary and secondary alcohols using an iron(III)/phenanthroline complex Eur. J. Inorg. Chem. 4479
Feig A L and Lippard S J 1994 Reactions of non-heme iron (II) centers with dioxygen in biology and chemistry Chem. Rev. 94 759
(a) Chowdhury B, Maji M and Biswas B 2017 Catalytic Aspects of a Copper(II) Complex: biological oxidase to oxygenase activity J. Chem. Sci. 129 1627
(a) Lipscomb D and Orville A M 1992 Degradation of Environmental Pollutants by Microorganisms and Their Metalloenzymes H Sigel and A Sigel A (Eds.) (New York: Marcel Dekker) Vol. 28 p. 243; (b) Mayilmurugan R, Visvaganesan K, Suresh E and Palaniandavar M 2009 Iron(III) Complexes of tridentate 3N ligands as functional models for catechol dioxygenases: the role of ligand \(N\)-alkyl substitution and solvent on reaction rate and product selectivity Inorg. Chem. 48 8771; (c) Dey D and Biswas B 2016 Catechol dioxygenase activity of a mononuclear iron(III)-phenanthroline complex J. Indian Chem. Soc. 93 495
(a) Dey D, De A, Yadav H R, Guin P S, Choudhury A R, Kole N and Biswas B 2016 An Oxo-Bridged Diiron(II) Complex as functional model of catechol dioxygenase ChemistrySelect 01 1910; (b) De A, Garai M, Yadav H R, Choudhury A R and Biswas B 2017 Catalytic promiscuity of an iron(II)-phenanthroline complex Appl. Organometal. Chem. 31 1
(a) Spence E L, Kawamukai M, Sanvoisin J, Braven H and Bugg T D H 1996 Catechol dioxygenases from Escherichia coli (MhpB) and Alcaligenes eutrophus (MpcI): sequence analysis and biochemical properties of a third family of extradiol dioxygenases J. Bacteriol. 178 5249; (b) Chatterjee S, Sheet D and Paine T K 2013 Catalytic and regiospecific extradiol cleavage of catechol by a biomimetic iron complex Chem. Commun. 49 10251; (c) Balamurugan M, Vadivelu P and Palaniandavar M 2014 Iron (III) complexes of tripodal tetradentate 4N ligands as functional models for catechol dioxygenases: the electronic vs. steric effect on extradiol cleavage Dalton Trans. 43 14653
(a) Bossek U, Hummel H, Weyhermüller T, Bill E and Wieghardt K 1995 The first \(\mu \) (OH)-bridged model complex for the mixed- valent \(\text{Fe}^{{\rm II}}\text{ Fe }^{{\rm III}}\) form of hemerythrin Angew. Chem. Int. Ed. Engl. 34 2642; (b) Crawford R L, Olson P E and Frick T D 1979 Catabolism of 5-chlorosalicylate by a Bacillus isolated from the Mississippi River Appl. Environ. Microbiol. 38 379
(a) Adachi K, Iwabuchi T, Sano H and Harayama S 1999 Structure of the ring cleavage product of 1-hydroxy-2-naphthoate, an intermediate of the phenanthrene-degradative pathway of Nocardioides sp. strain KP7 J. Bacteriol. 181 757; (b) Hintner J P, Lechner C, Riegert U, Kuhm A E, Storm T, Reemtsma T and Stolz A 2001 Direct ring fission of salicylate by a salicylate 1,2-dioxygenase activity from Pseudaminobacter salicylatoxidans J. Bacteriol. 183 6936
(a) Ladd J N 1962 Oxidation of anthranilic acid by a species of Achromobacter isolated from soil Nature 194 1099; (b) Walker N and Lippert K D 1965 Formation of gentisic acid from 2-napthol by a Pseudomonas. Biochem. J. 95 5C; (c) Crawford R L 1975 Degradation of 3-hydroxybenzoate by bacteria of the genus Bacillus. Appl. Microbiol. 30 439; (d) Crawford R L 1976 Pathways of 4-hydroxybenzoate degradation among species of Bacillus. J. Bacteriol. 127 204; (e) Tomasek P H and Crawford R L 1986 Initial reactions of xanthone biodegradation by an Arthrobacter sp. J. Bacteriol. 167 818; (f) Yano K and Arima K 1958 Metabolism of aromatic compounds by bacteria, II. m-Hydroxybenzoic acid hydroxylase A and B; 5-dehydroshikimic acid, a precursor of protocatechuic acid: a new pathway from salicylic acid to gentisic acid J. Gen. Appl. Microbiol. 4 241
Iwabuchi T and Harayama S 1998 Biochemical and molecular characterization of 1-hydroxy-2-naphthoate dioxygenase from Nocardioides sp. KP7 J. Biol. Chem. 273 8332
Hintner J P, Reemtsma T and Stolz A 2004 Biochemical and molecular characterization of a ring fission dioxygenase with the ability to oxidize (substituted) salicylate(s) from Pseudaminobacter salicylatoxidans J. Biol. Chem. 279 37250
Lack L 1959 The enzymic oxidation of gentisic acid Biochim. Biophys. Acta 34 117
Knox E and Edwards S W 1955 The properties of maleylacetate, the initial product of homogentisate oxidation in liver J. Biol. Chem. 216 489
Harpel M R and Lipscomb J D 1990 Gentisate 1,2-dioxygenase from pseudomonas. Purification, characterization, and comparison of the enzymes from Pseudomonas testosteroni and Pseudomonas acidovorans J. Biol. Chem. 265 6301
Miyauchi K, Adachi Y, Nagata Y and Takagi M 1999 Cloning and sequencing of a novel meta-cleavage dioxygenase gene whose product is involved in degradation of gamma-hexachlorocyclohexane in Sphingomonas paucimobilis J. Bacteriol. 181 6712
Veldhuizen E J, Vaillancourt F H, Whiting C J, Hsiao M M, Gingras G and Xiao Y 2005 Steadystate kinetics and inhibition of anaerobically purified human homogentisate 1,2-dioxygenase Biochem. J. 386 305
Harpel M R and Lipscomb J D 1990 Gentisate 1,2-dioxygenase from Pseudomonas. Substrate coordination to active site \(\text{ Fe }^{2+}\) and mechanism of turnover J. Biol. Chem. 265 22187
Vaillancourt F H, Bolin J T and Eltis L D 2006 The ins and outs of ring-cleaving dioxygenases Crit. Rev. Biochem. Mol. Biol. 41 241
Gütlich P, Garcia Y and Goodwin H A 2000 Spin crossover phenomena in Fe(II) complexes Chem. Soc. Rev. 29 419
Childs B J, Craig D C, Scudder M L and Goodwin H A 1998 Structural and electronic studies of the coordination of 6-(thiazol-2-yl)-2, 2\(^{\prime }\)-bipyridine and related systems to Fe(II), Co(II) and Ni(II) Inorg. Chim. Acta 274 32
CrystalClear 2.0; Rigaku Corporation: Tokyo, Japan
Sheldrick G M 2008 Crystal structure refinement with SHELXL Acta Cryst. A64 112
Dolomanov O V, Bourhis L J, Gildea R J, Howard J A K and Puschmann H 2009 OLEX2: A complete structure solution, refinement and analysis program J. Appl. Cryst. 42 339
Sawyer D T 1991 Oxygen Chemistry (New York: Oxford University Press)
Halder P, Paria S and Paine T K 2012 Dioxygen reactivity of biomimetic iron–catecholate and iron–o-aminophenolate complexes of a tris(2-pyridylthio)methanido ligand: Aromatic CC Bond cleavage of catecholate versus o-iminobenzosemiquinonate radical formation Chem. Eur. J. 18 11778
Nakamoto K 1997 Infrared and Raman Spectra of Inorganic and Coordination Compounds part B: Applications in Coordination, Organometallic and Bioinorganic Chemistry (New York: John Wiley & Sons Inc.)
Solé J G, Bausá L E and Jaque D 2005 An Introduction to Optical Spectroscopy of Inorganic Solids (UK: John Wiley & Sons Ltd.)
Biswas B and Sengupta P S 2017 An iron(III)-Schiff base complex as a functional model of phenoxazinone synthase enzyme J. Indian Chem. Soc. 94 571
(a) Wang Q X, Jiao K W, Sun F F and Jian X H 2006 Recent developments in recyclable copper catalyst systems for C–N bond formingcross-coupling reactions using aryl halides and arylboronic acids Eur. J. Inorg. Chem. 1838; (b) Dey D, Das S, Yadav H R, Ranjani A, Gyathri L, Roy S, Guin P S, Dhanasekaran D, Choudhury A R, Akbarsha M A and Biswas B 2016 Design of a mononuclear copper(II)-phenanthroline complex: Catechol oxidation, DNA cleavage and antitumor properties Polyhedron 106 106; (c) De A, Dey D, Yadav H R, Maji M, Rane V, Kadam R M, Roy Choudhury A and Biswas B 2016 Unprecedented hetero-geometric discrete copper(II) complexes: Crystal structure and bio-mimicking of Catecholase activity J. Chem. Sci. 128 1775
(a) Tian Y P, Duan C Y, Xu X X and You X Z 1995 Screw-chain structure of 1,10-phenanthroline hydrate, \(\text{ C }_{12}\text{ H }_{8}\text{ N }_{2}.\text{ H }_{2}\text{ O }\) Acta Crystallogr. Sect. C 51 2309; (b) Dey D, Roy A B, Shen C -Y, Tsai H -L, Ranjani A, Gayathri L, Chandraleka S, Dhanasekaran D, Akbarsha M A, Kole N and Biswas B 2015 Synthesis and bio-catalytic activity of isostructural cobalt(III)-phenanthroline complexes J. Chem. Sci. 127 649; (c) Garai M, Dey D, Maji M, Yadav H R, Choudhury A R and Biswas B 2017 Synthesis and phosphatase activity of a cobalt(II)-phenanthroline complex J. Chem. Sci. 129 1513
E Westhoff (Ed.) 1993 Water and Biological Macromolecules (Boca Raton: CRC Press)
(a) Das S, Pasan J, Ranjani A, Gayathri L, Saha S, Chandraleka S, Dhanasekaran D, Pasan J, Maji M, Akbarsha M A and Biswas B 2016 Recognition of self-assembled water-nitrate cluster in a Co(III)-2,2’-bipyridine host: Synthesis, crystal structure, DNA cleavage, molecular docking and anticancer activity J. Chem. Sci. 128 1755; (b) Dey D, Pal S, Yadav H R, Sengupta P S, A R Choudhury, Kole N and Biswas B 2015 Unusual crystallographic existence of hydrated zinc(II) bisulphate complex: An experimental & theoretical observations RSC Adv. 5 42681
(a) Stolz A, Nörtemann B and Knackmuss H J 1992 Bacterial metabolism of 5-aminosalicylic acid. Initial ring cleavage Biochem. J. 282 675; (b) Lendenmann U and Spain J C 1996 2-aminophenol 1,6-dioxygenase: a novel aromatic ring cleavage enzyme purified from Pseudomonas pseudoalcaligenes JS45 J. Bacteriol. 178 6227; (c) Kurahashi T, Oda K, Sugimoto M, Ogura T and Fujii H 2006 Trigonal-bipyramidal geometry induced by an external water ligand in a sterically hindered iron salen complex, related to the active site of protocatechuate 3,4-dioxygenase Inorg. Chem. 45 7709; (d) Merkel M, Müller F K and Krebs B 2002 Novel iron (III) complexes with phenolate containing tripodal tetradentate ligands as model systems for catechol 1,2-dioxygenases Inorg. Chim. Acta 337 308
Pascaly M, Duda M, Schweppe F, Zurlinden F, Muller K and Krebs B 2001 The systematic influence of tripodal ligands on the catechol cleaving activity of iron (III) containing model compounds for catechol 1,2-dioxygenases J. Chem. Soc. Dalton Trans. 828
Koch W O and Kruger H J 1995 A highly reactive and catalytically active model system for intradiol-cleaving catechol dioxygenases: structure and reactivity of iron(III) catecholate complexes of \(N,N^{\prime }\)-dimethyl-2,11-diaza[3.3](2,6)pyridinophane Angew. Chem. Int. Ed. Engl. 34 2671
(a) Takenaka S, Murakami S, Shinke R, Hatakeyama K, Yukawa H and Aoki K 1997 Novel genes encoding 2-aminophenol 1,6-dioxygenase from Pseudomonas species AP-3 growing on 2-aminophenol and catalytic properties of the purified enzyme J. Biol. Chem. 272 14727; (b) Ito M and Que L Jr 1997 Biomimetic extradiol cleavage of catechols: Insights into the enzyme mechanism Angew. Chem. Int. Ed. Engl. 36 1342; (c) Cox D, Benkovic S J, Bloom L M, Bradley F C, Nelson M J, Que L Jr. and Wallick D E 1988 Catecholate LMCT bands as probes for the active sites of nonheme iron oxygenases J. Am. Chem. Soc. 110 2026
(a) Muraki T, Taki M, Hasegawa Y, Iwaki H and Lau P C 2003 Prokaryotic homologs of the eukaryotic 3-hydroxyanthranilate 3,4-dioxygenase and 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase in the 2-nitrobenzoate degradation pathway of Pseudomonas fluorescens strain KU-7 Appl. Environ. Microbiol. 69 1564; (b) Funabiki T, Mizoguchi A, Sugimoto T, Tada S, Tsuji M, Sakamoto H and Yoshida S 1986 Oxygenase model reactions: Intra-and extradiol oxygenations of 3,5-di-tert-butylcatechol catalyzed by (bipyridine)(pyridine)iron(III) complex J. Am. Chem. Soc. 108 2921; (c) Die A, Gatteschi D and Pardi L 1993 Synthesis, characterization, and reactivity of catecholato adducts of iron(III) triaza- and tetraazamacrocyclic complexes: chemical evidence of the role of the metal ion in the oxidative cleavage Inorg. Chem. 32 1389
Acknowledgements
BB gratefully acknowledges the financial support by the Science & Engineering Research Board (SERB), Department of Science and Technology (DST), New Delhi, India under FAST TRACK SCHEME for YOUNG SCIENTIST (No. SB/FT/CS-088/2013 dtd. 21/05/2014).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De, A., Dey, D., Das, A. et al. Gentisate-1,2-dioxygenase activity by an iron(II)-phenanthroline complex. J Chem Sci 130, 26 (2018). https://doi.org/10.1007/s12039-018-1425-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-018-1425-4