Abstract
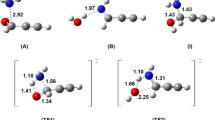
The cycloaddition reaction mechanism between interstellar molecules, ketenimine and methyleneimine, has been systematically investigated employing the second-order Møller-Plesset perturbation theory (MP2) method in order to better understand the reactivity of nitrogenous cumulene ketenimine with the C =N double bond compound methyleneimine. Geometry optimizations and vibrational analyses have been performed for the stationary points on the potential energy surfaces of the system. Calculations show that five-membered cyclic carbene intermediates could be produced through pericyclic reaction processes between ketenimine and methyleneimine. Through the subsequent hydrogen transfer processes, carbene intermediates can be isomerized to the pyrazole and imidazole compounds, respectively. The present study is helpful to understand the formation of prebiotic species in interstellar space.

The cycloaddition reaction mechanism between interstellar molecules, ketenimine and methyleneimine, has been systematically investigated theoretically. The products of this reaction are pyrazole and imidazole compounds, respectively.






Similar content being viewed by others
References
Cláudio M N, Igor R, Teresa M D, Pinhoe M, Rui F, Tomáš Š and Thomas B 2011 J. Am. Chem. Soc. 50 18911
Lovas F J, Hollis J M, Remijan A J and Jewell P R 2006 Astrophys. J. 2 L137
Jacox M E and Milligan D E 1963 J. Am. Chem. Soc. 3 278
Jacox M E 1979 Chem. Phys. 2 157
Rodler M, Brown R D, Godfrey P D and Tack L M 1984 Chem. Phys. Lett. 5 447
Nadia B, Dimitrios S, Francesca L, Raffaele P, Mathias H, Wolf D G and Piergiorgio C 2012 J. Phys. Chem. A 43 10467
Hudson R L and Moore M H 2004 Icarus 172 466
Lamsabhi A M, Otilia M, Manuel Y, Salpin J Y, Haldys V, Tortajada J and Guillemin J C 2008 J. Phys. Chem. A 42 10509
Guennoun Z, Couturier-Tamburelli I, Combes S, Aycard J P and Piétri N 2005 J. Phys. Chem. A 51 11733
Liu S, Lei Y, Qi X T and Lan Y 2014 J. Phys. Chem. A 14 2638
Fang D C and Li H M 2000 J. Mol. Struc. (Theochem) 528 111
Sun X M, Wei X G, Wu X P, Ren Y, Wong N B and Li W K 2010 J. Phys. Chem. A 1 595
Sung K, Wu S H, Wu R R and Sun S Y 2002 J. Org. Chem. 12 4298
Wu F and He S R 2000 Acta Phys-Chim. Sin. 16 243
Hollis J M, Remijan A J, Jewell P R and Lovas F J 2006 Astrophys. J. 2 933
Ikeda M, Ohishi M, Nummelin A, Dickens J E, Bergman P, Hjalmarson Å and Irvine W M 2001 Astrophys. J. 2 792
Bottinelli S, Ceccarelli C, Williams J P and Lefloch B 2007 Astron. Astrophys. 2 601
Scott A S, Jérôme A, Conel M, Tohru A, Saša B, Giuseppe A B, Janet B, John P B, Donald E B, John R B, Mark J B, Henner B, Anna B, Simon J C, George C, Luigi C, George C, Louis D, Zahia D, Jason P D, Gianluca F, Holger F, George J F, Ian A F, Marc F., Mary K G, Daniel P G, Matthieu G, Faustine G, Chris J, Lindsay P K, Kilcoyne A L, Jan L, Matrajt G, Meibom A, Mennella V, Mostefaoui S, Nittler L R, Palumbo M E, Papanastassiou D A, Robert F, Rotundi A, Snead C J, Spencer M K, Stadermann F J, Steele A, Stephan T, Tsou P, Tyliszczak T, Westphal A J, Wirick S, Wopenka B, Yabuta H, Zare R N and Zolensky M E 2006 Science 314 1720
Pizzarello S, Huang Y, Becker L, Poreda R J, Nieman R A, Cooper G and Williams M 2001 Science 293 2236
Remijan A J, Hollis J M, Lovas F J, Plusquellic D F and Jewell P R 2005 Astrophys. J. 632 333
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Zakrzewski V G, Montgomery J A, Stratmann R E, Burant J C, Dapprich S, Millam J M, Daniels A D, Kudin K N, Strain M C, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson G A, Ayala P Y, Cui Q, Morokuma K, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Cioslowski J, Ortiz J V, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Gonzalez C, Challacombe M, Gill P M, Johnson B G, Chen W, Wong M W, Andres J L, Head-Gordon M, Replogle E S and Pople J A 1998 Gaussian 98, Revision A.9 (Gaussian Inc., Pittsburgh, PA)
Lattelais M, Pauzat F, Ellinger Y and Ceccarelli C 2009 Astrophys. J. 696 L133
Acknowledgements
This work is supported by NSFC (21003082, 21303093, 21577076, 21403088), the NSF of Shandong Province (ZR2014BM020), and the project of Shandong Province Higher Educational Science and Technology Program (J13LM06). The State Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences (KF2013-05) are also acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supporting Information (SI)
Atomic coordinates and other data for the molecules are available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
WANG, N., TAN, X., WANG, W. et al. Theoretical insights into the cycloaddition reaction mechanism between ketenimine and methyleneimine: An alternative approach to the formation of pyrazole and imidazole. J Chem Sci 128, 279–285 (2016). https://doi.org/10.1007/s12039-015-1028-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-1028-2