Abstract
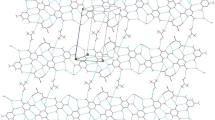
Crystals of 7,11-bis(2,4-dichlorophenyl)-2,4-dimethyl-2,4-diazaspiro[5.5]undecane -1,3,5,9-tetraone were grown in polar solvents and subjected to single crystal X-ray diffraction. The molecular crystal is Triclinic, P-1, a= 8.3734 (19) Å, b= 12.382 (3) Å, c= 12.871 (3) Å, α= 66.639 (7) ∘, β= 85.148 (7) ∘, γ= 70.690 (6) ∘, V= 1154.5 (5)Å 3, Z= 2, D calc= 1.519 g cm −3. The optimized molecular structure of the studied compound using B3LYP/6-311G(d,p) method showed good agreement with the X-ray structure. The electronic and spectroscopic properties of the title compound were predicted. The NBO calculations were used to calculate the natural atomic charges at the different atomic sites as well as the intramolecular charge transfer (ICT) interactions among the most significant natural orbitals. The high LP(N) → BD*(2)C-O ICT interaction energies indicate strong electron delocalization from the lone pair of the N-atoms of the pyrimidinetrione ring to the adjacent carbonyl groups. In contrast, the small LP(O) →BD*(1)C-H stabilization energies (E (2)) indicated weak C-H—O interactions. Experimentally, the studied compound showed the most intense electronic transition band at 232 nm which is calculated using TD-DFT method as a shoulder at 231.3 nm (f =0.0832) and it belongs to H-3/H-1 →L+1 and H-2 →L+2 excitations. The GIAO calculated 1H and 13C NMR chemical shifts showed good correlations with the experimental data.

The X-Ray single crystal structure and DFT computation of the 7,11-bis(2,4-dichlorophenyl)-2,4-dimethyl-2,4-diazaspiro[5.5]undecane -1,3,5,9-tetraone are discussed.









Similar content being viewed by others
References
(a) Kotha S, Deb A C, Lahiri K and Manivannan E 2009 Synthesis 2009 165; (b) Bartoli A, Rodier F, Commeiras L, Parrain J L and Chouraqui G 2011 Nat. Prod. Rep. 28 763
Pradhan R, Patra M, Behera A K, Mishra B K and Behera R K 2006 Tetrahedron 62 779
(a) Saragi T P I, Spehr T, Siebert A, Lieker T F and Salbeck J 2007 Chem. Rev. 107 1011; (b) Xie J H and Zhou Q L 2008 Acc. Chem. Res. 41 581; (c) Ding K, Han Z and Wang Z 2009 Chem.-Asian J. 4 32
Karatholuvhu M S, Sinclair A, Newton A F, Alcaraz M L, Stockman R A and Fuchs P L 2006 J. Am. Chem. Soc. 128 12656
Taylor E C, Berrier J V, Cocuzza A J, Kobylecki R and McCormack J J 1977 J. Med. Chem. 20 1215
Naydenova E, Pencheva N, Popova J, Stoyanov N, Lazarova M and Aleksiev B 2002 Il Farmaco 57 189
Takahashi S K, Witkop B, Brossi A, Maleque M A and Albuquerque E X 1982 Helv. Chim. Acta. 65 252
Kim H S, Nagai Y, Ono K, Begum K, Wataya Y, Hamada Y, Tsuchiya K, Masuyama A, Nojima M and McCullough K J 2001 J. Med. Chem. 44 2357
Reddy D M, Qazi N A, Sawant S D, Bandey A H, Srinivas J, Shankar M, Singh S K, Verma M, Chashoo G, Saxena A, Mondhe D, Saxena A K, Sethi V K, Taneja S C, Qazi G N and Kumar H M S 2011 Eur. J. Med. Chem. 46 3210
(a) Srivastav N, Mittal A and Kumar A, 1992 J. Chem. Soc. 493; (b) Longeon A, Guyot M, Vacelet J 1990 Experentia 46 548
Osman A N, Kandeel M M, Said M M and Ahmed E M 1996 Indian J. Chem. Sect. B: Org. Chem. Incl. Med. Chem. 35 1073
Behera R K, Behera A K, Pradhan R, Pati A and Patra M 2009 Phosphorus, Sulfur Silicon Relat. Elem. 184 753
Kesharwani S, Sahu N K and Kohli D V 2009 Pharm. Chem. J. 43 315
Goel B, Sharma S, Bajaj K, Bansal D, Singh T, Malik N, Lata S, Tyagi C, Panwar H, Agarwal A and Kumar A 2005 Indian J. Pharm. Sci. 67 194
Behera R K, Behera A K, Pradhan R, Pati A and Patra M 2006 Synth. Commun. 36 3729
(a) Theford D, Chorton A P and Hardman J 2003 Dyes Pigm. 59 185; (b) Karci F 2008 Dyes Pigm. 77 451; (c) Wang S and Kim S H 2009 Dyes Pigm. 80 314
(a) Michael A J 1887 Prakt Chem. 35 349; (b) Jung M E 1991 In Comprehensive Organic Synthesis Vol. 4 B M Trost, I Fleming and M F Semmelhack (Eds.) (Oxford: Pergamon) pp 1–68; (c) Perlmutter P 1992 In Conjugate Addition Reactions in Organic Synthesis (New York: Elsevier Science); (d) Barakat A, Islam M S, Al Majid A M A and Al-Othman Z A 2013 Tetrahedron 69 5185; (e) Islam M S, Al Majid A M A, Al-Othman Z A, Barakat A 2014 Tetrahedron: Asymmetry 25 245; (f) Al Majid A M A, Islam M S, Barakat A, Al-Agamy M H M and Naushad M 2014 The Scientific World Journal, doi:10.1155/2014/649197; (g) Barakat A, Islam M S, Al-Majid A M, Ghabbour H A, Fun H-K, Javed K, Imad R, Yousuf S, Choudhary M I and Wadood A 2015 Bioorg. Med. Chem. 23 740; (h) Barakat A, Al Majid A M, Islam M S, Soliman S M, Siddiqui M R H, Ghabbour H A and Fun H-K 2015 J. Chem. Sci. doi:10.1007/s12039-015-0923-x
Sheldrick G M 2008 Acta Cryst. A 64 112
Spek A L 2009 Acta Cryst. D 65 148
(a) www.gaussian.com; (b) Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A and Cheeseman J R Gaussian-03, Revision C.01 2004 (Wallingford, CT, USA: Gaussian, Inc.)
Dennington R II, Keith T and Millam J, Gauss View, Version 4.1.2 2007 (Shawnee Mission, KS, USA: Semichem Inc.)
Zhurko G A and Zhurko D A 2005 ChemCraft: Tool for Treatment of Chemical Data, Late version build 08 (freeware). http://www.chemcraftprog. com (accessed on 5 May 2015)
Glendening E D, Reed A E, Carpenter J E and Weinhold F 1998 NBO Version 3.1 TCI (Madison, WI, USA: University of Wisconsin)
Islam M S, Barakat A, Al Majid A M A, Ghabbour H A, Fun H-K and Siddiqui M R 2015 Arab. J. Chem. doi:10.1016/j.arabjc. 2015.03.007
Murray J S and Sen K 1996 In Molecular Electrostatic Potentials, Concepts and Applications (Amsterdam: Elsevier)
Scrocco E and Tomasi J 1978 Adv. Quantum. Chem. 11 115
Fukui K, Yonezawa T and Shingu H J 1952 J. Chem. Phys. 20 722
Padmaja L, Ravikumar C, Sajan D, Joe I H, Jayakumar V S, Pettit G R and Neilsen F O 2009 J. Raman Spectrosc. 40 419
Ravikumar C, Joe I H and Jayakumar V S 2008 Chem. Phys. Lett. 460 552
Pearson R G 1989 J. Org. Chem. 54 1430
Parr R G and Pearson R G 1983 J. Am. Chem. Soc. 105 7512
Geerlings P, De-Proft F and Langenaeker W 2003 Chem. Rev. 103 1793
Parr R G, Szentpaly L and Liu S 1999 J. Am. Chem. Soc. 121 1922
Chattaraj K and Giri S 2007 J. Phys. Chem. A 111 11116
Parr R G, and Yang W 1989 In Density-Functional Theory of Atoms and Molecules (New York: Oxford University Press)
Singh R N, Kumar A, Tiwari R K, Rawat P and Gupta V P 2013 J. Mol. Struct. 1035 427
Joe I H, Kostova I, Ravikumar C, Amalanathan M and Pinzaru S C 2009 J. Raman Spectrosc. 40 1033
Sebastian S and Sundaraganesan N 2010 Spectro. Chim. Acta Part A 75 941
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this Research group NO (RG -044-1435-1436).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
All additional information pertaining to characterization of the target compound: The correlation graphs between calculated and observed geometrical parameters of the title compound are shown in figure S1 S1. NMR (figures S2 and S3), IR (figure S4), H-bonding information (table S1) and NMR chemical shift data (table S2) are given in the Supplementary Information which is available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
ISLAM, M.S., BARAKAT, A., AL-MAJID, A.M. et al. Crystal Structure of 7,11-bis(2,4-dichlorophenyl)-2,4-dimethyl-2,4- diazaspiro[5.5]undecane -1,3,5,9-tetraone and its computational studies. J Chem Sci 127, 2039–2050 (2015). https://doi.org/10.1007/s12039-015-0969-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0969-9