Abstract
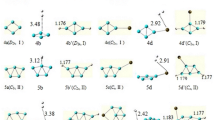
Adsorption of CO, N2, H2O, O2, H2 and NO molecules on B36 cluster was studied using density functional theory (DFT) with B3LYP functional and 6-311 + G(d,p) basis set. Energies, enthalpies and Gibbs free energies of the adsorption processes were calculated. The thermodynamic data showed that the B36 cluster is a good adsorbent only for CO, O2 and NO molecules. The calculated energies of adsorption of N2, H2O and H2 on the B36 cluster were positive values. CO molecule is adsorbed via the carbon atom more effectively, while the nitrogen atom of NO is adsorbed better than the oxygen atom. Also, when NO and O2 are adsorbed synchronously via both atoms, they dissociate. The edge boron atoms of the B36 cluster showed more reactivity than the inner atoms.

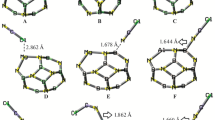
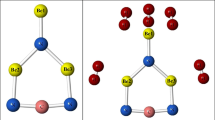
B36 cluster is a single-atom thick boron cluster which has both planar and quasi-planar structures. When NO molecule is adsorbed on the inner boron atoms of the B36 cluster, a B-B bond is broken and N-B and O-B bonds are formed (A). Adsorption of NO molecule on the outer (edge) boron atoms of the B36 cluster is a dissociative adsorption (B).







Similar content being viewed by others
References
Alexandrova A N, Boldyrev A I, Zhai H J and Wang L S 2006 Coord. Chem. Rev. 250 2811
Li S D, Guo J C, Miao C Q and Ren G M 2005 Angew. Chem. Int. Ed. 44 2158
Chen Q, Zhang S Y, Bai H, Tian W J, Gao T, Li H R, Miao C Q, Mu Y W, Lu H G, Zhai H J and Li S D 2015 Angew. Chem. Int. Ed. 54 8160
Xu J, Chan Y, Gan L, Ma Y and Zhai T 2015 Adv. Sci. 2. doi: 10.1002/advs.201500023
Prasad D L V K and Jemmis E D 2008 Phys. Rev. Lett. 100 165504
Zhai H J, Zhao Y F, Li W L, Chen Q, Bai H, Hu H S, Piazza Z A, Tian W J, Lu H G, Wu Y B, Mu Y W, We G F, Liu Z P, Li J, Li S D and Wang L S 1999 Nat. Chem. 6 727
Szwacki N G, Sadrzadeh A and Yakobson B I 2008 Phys. Rev. Lett. 98 166804
Boustani I, Quandt A, Hernandez E and Rubio A 1999 J. Chem. Phys. 110 3176
Sergeeva A P, Popov I A, Piazza Z A, Li W L, Romanescu C, Wang L S and Boldyrev A I 2014 Acc. Chem. Res. 47 1349
Galeev T R, Chen Q, Guo J C, Bai H, Miao C Q, Lu H G, Sergeeva A P, Li S D and Boldyrevn A I 2011 Phys. Chem. Chem. Phys. 13 11575
Piazza Z A, Li W L, Romanescu C, Sergeeva A P, Wang L S and Boldyrev A 2012 J. Chem. Phys. 136 104310
Yang X, Ding Y and Ni J 2008 Phys. Rev. B 77 041402 (R)
Tang H and Ismail-Beigi S 2007 Phys. Rev. Lett. 99 115501
Sergeeva A P, Zubarev D Y, Zhai H J, Boldyrev A I and Wang L S 2008 J. Am. Chem. Soc. 130 7244
Zhai H J, Kiran B, Li J and Wang L S 2003 Nat. Mater. 2 827
Sergeeva A P, Piazza Z A, Romanescu C, Li W L and Boldyrev A I 2012 J. Am. Chem. Soc. 134 18065
Huang W, Sergeeva A P, Zhai H J, Averkiev B B, Wang L S and Boldyrev A I 2010 Nat. Chem. 2 202
Wu X, Dai J, Zhao Y, Zhao Z, Yang J and Zeng X C 2012 ACS Nano 6 7443
Sun Q, Wang M, Li Z, Du A and Searles D J 2014 J. Phys. Chem. C 118 2170
Sun Q, Wang M, Li Z, Du A and Searles D J 2014 Phys. Chem. Chem. Phys. 16 12695
Piazza Z A, Hu H S, Li W L, Zhao Y F, Li J and Wang L S 2014 Nat. Commun. 5 3113
Chen Q, Wei G F, Tian W J, Bai H, Liu Z P, Zhai H J and Li S D 2014 Phys. Chem. Chem. Phys. 16 18282
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Scalmani G, Barone V, Mennucci B, Petersson G A, Nakatsuji H, Caricato M, Li X, Hratchian H P, Izmaylov A F, Bloino J, Zheng G, Sonnenberg J L, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J A, Jr., Peralta J E, Ogliaro F, Bearpark M, Heyd J J, Brothers E, Kudin K N, Staroverov V N, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant J C, Iyengar S S, Tomasi J, Cossi M, Rega N, Millam J M, Klene M, Knox J E, Cross J B, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Martin R L, Morokuma K, Zakrzewski V G, Voth G A, Salvador P, Dannenberg J J, DapprichS, Daniels A D, Farkas O, Foresman J B, Ortiz J V, Cioslowski J and Fox D J 2009 Gaussian 09, Revision A.1. (Wallingford: Gaussian, Inc.)
Nojini Z B and Samiee S 2011 J. Phys. Chem. C 115 12054
Rafati A A, Hashemianzadeh S M and Nojini Z B 2008 J. Phys. Chem. C 112 3597
Crabtree R H 2005 In The Organometallic Chemistry of Transition Metals (New York: John Wiley)
Fujitani T, Nakamura L, Kobayashi Y, Takahashi I, Haneda M and Hamada H 2005 J. Phys. Chem. B 109 17603
Machida M, Ikeda S, Kurogi D and Kijima T 2001 Appl. Catal. A 35 107
Ertl G, Lee S B and Weiss M 1982 Surf. Sci. 114 515
Acknowledgment
The authors wish to express thanks to the Center of Excellency in Chemistry of Isfahan University of Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
All additional information pertaining to geometrical parameters of the compounds and their charge distribution analysis (figures 1, 2) are given in the supporting information available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
VALADBEIGI, Y., FARROKHPOUR, H. & TABRIZCHI, M. Adsorption of small gas molecules on B36 nanocluster. J Chem Sci 127, 2029–2038 (2015). https://doi.org/10.1007/s12039-015-0967-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0967-y