Abstract
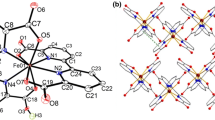
Reactions of CeCl3⋅6H2O and Gd(NO3)3⋅5H2O with biacetyl bis(benzoylhydrazone) (H2babh) and KOH in 1:2:2 mole ratio in methanol afford the complexes [Ce(babh)2] (1) and [Gd(babh)(Hbabh)]⋅H2O (2⋅H2O), respectively in good yields. Characterization of the complexes has been performed with the help of elemental analysis, magnetic susceptibility, spectroscopic (IR, UV-Vis, EPR and NMR) and X-ray crystallographic measurements. 1 is diamagnetic and NMR active, while 2⋅H2O is paramagnetic (μ eff = 8.03 μ B at 300 K) and EPR active. The complexes crystallize as 1⋅CH2Cl2 and 2⋅H2O. X-ray structures show that the metal centre in each of 1 and 2 is in a distorted dodecahedral N4O4 coordination sphere assembled by two meridionally spanning ONNO-donor ligands. Self-assembly of 1⋅CH2Cl2 via intermolecular C−H⋯N and C−H⋯Cl hydrogen bonds and π- π interactions provides one-dimensional ‘ladder’ type structure. On the other hand, 2⋅H2O assembles into a two-dimensional ‘sheet’ like network through intermolecular N−H⋯O and O−H⋯N hydrogen bonds.

Two octacoordinated complexes [Ce(babh)2] and [Gd(babh)(Hbabh)] (H2babh = biacetyl bis(benzoylhydrazone)) are reported. They are characterized by microanalytical, crystallographic, spectroscopic and magnetic measurements. The meridional ONNO-donor ligands assemble a distorted dodecahedral N4O4 coordination sphere in each complex. Selfassembly of the solvated complexes via intermolecular non-covalent interactions generates 1D ladder and 2D sheet structures.








Similar content being viewed by others
References
Parker D, Dickins R S, Puschmann H, Crossland C and Howard J A K 2002 Chem. Rev. 102 1977
Cotton S A 2005 C. R. Chimie 8 129
Tsukube H, Shinoda S and Tamiaki H 2002 Coord. Chem. Rev. 226 227
Carr R, Evans N H and Parker D 2012 Chem. Soc. Rev. 41 7673
Hussain A and Chakravarty A R 2012 J. Chem. Sci. 124 1327
dos Santos C M G, Harte A J, Quinn S J and Gunnlaugsson T 2008 Coord. Chem. Rev. 252 2512
Brunet E, Juanes O and Rodriguez-Ubis J C 2007 Curr. Chem. Biol. 1 11
Faulkner S, Pope S J A and Burton-Pye B P 2005 Appl. Spectrosc. Rev. 40 1
Armelaoa L, Quici S, Barigelletti F, Accorsi G, Bottaro G, Cavazzini M and Tondello E 2010 Coord. Chem. Rev. 254 487
Binnemans K 2009 Chem. Rev. 109 4283
Feng J and Zhang H 2013 Chem. Soc. Rev. 42 387
Sessoli R and Powell A K 2009 Coord. Chem. Rev. 253 2328
Luzon J and Sessoli R 2012 Dalton Trans 41 13556
Zhang P, Guo Y-N and Tang J 2013 Coord. Chem. Rev. 257 1728
Habib F and Murugesu M 2013 Chem. Soc. Rev. 42 3278
Aime S, Botta M and Terreno E 2005 Adv. Inorg. Chem. 57 173
Woods M, Woessner D E and Sherry A D 2006 Chem. Soc. Rev. 35 500
Aime S, Crich S G, Gianolio E, Giovenzana G B, Tei L and Terreno E 2006 Coord. Chem. Rev. 250 1562
Werner E J, Datta A, Jocher C J and Raymond K N 2008 Angew. Chem. Intl. Ed. 47 8568
Shibasaki M and Yoshikawa N 2002 Chem. Rev. 102 2187
Li H-X, Zhu Y-J, Cheng M-L, Ren Z-G, Lang J-P and Shen Q 2006 Coord. Chem. Rev. 250 2059
Visseaux M and Bonnet F 2011 Coord. Chem. Rev. 255 374
Ghosh T, Mukhopadhyay A, Dargaiah K S C and Pal S 2010 Struct. Chem. 21 147
Ghosh T and Pal S 2010 Inorg. Chim. Acta 363 3632
Bain G A and Berry J F 2008 J. Chem. Educ. 85 532
SMART Version 5.630 and SAINT-plus Version 6.45 2003 Bruker-Nonius Analytical X-ray Systems Inc., Madison, Wisconsin, USA
Sheldrick G M 1997 SADABS, Program for Area Detector Absorption Correction, University of Göttingen, Göttingen
Sheldrick G M 2008 Acta Crystallogr., Sect. A 64 112
Farrugia L J 1999 J. Appl. Crystallogr. 32 837
Macrae C F, Bruno I J, Chisholm J A, Edgington P R, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J and Wood P. A 2008 J. Appl. Crystallogr. 41 466
Spek A L 2002 Platon, A Multipurpose Crystallographic Tool Utrecht University, Utrecht, The Netherlands
Chen C, Chen H, Yan P and Hou G 2013 Li G Inorg. Chim. Acta 405 182
Sommerer S O, Westcott B L, Cundari T R and Krause J A 1993 Inorg. Chim. Acta 209 101
Benson M T, Cundari T R, Saunders L C and Sommerer S O 1997 Inorg. Chim. Acta 258 127
Kemp W 1987 In Organic Spectroscopy (Macmillan: Hampshire) pp. 62–66
Yatsimirskii K B and Davidenko N K 1979 Coord. Chem. Rev. 27 223
Prasad T K and Rajasekharan M V 2009 Inorg. Chem. 48 11543
Chakraborty J, Ray A, Pilet G, Chastanet G, Luneau D, Ziessel R F, Charbonnière L J, Carrella L, Rentschler E, El Fallahe M S and Mitra S 2009 Dalton Trans. 10263
Szyczewski A, Lis S, Kruczyński Z, Pietrzak J, But S and Elbanowski M 1996 Acta Phys. Pol. A 90 345
Akilan P, Thirumavalavan M and Kandaswamy M 2003 Indian J. Chem. Technol. 10 363
Acknowledgements
T. Ghosh thanks the University Grants Commission (UGC), New Delhi for a research fellowship. We thank the Department of Science and Technology (DST), New Delhi and the UGC, New Delhi for the facilities provided under the FIST and the CAS programmes, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
CCDC-1012274 and CCDC-1012275 contain the supplementary crystallographic data for 1⋅CH2Cl2 and 2⋅H2O, respectively. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Rights and permissions
About this article
Cite this article
GHOSH, T., PAL, S. Synthesis and characterization of dodecahedral cerium(IV) and gadolinium(III) complexes with a tetradentate Schiff Base. J Chem Sci 127, 1201–1209 (2015). https://doi.org/10.1007/s12039-015-0887-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0887-x