Abstract
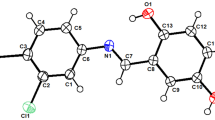
A series of novel amino acid derivatives has been synthesized by the reaction of 4-[4-methoxy-3-methylphenyl]-4-oxobutenoic acid with primary and secondary amines. The treatment of amino acids with hydrazine afforded pyridazine. Phenylhydrazone was obtained from the reaction of the acid with phenyl hydrazine in ethanol. On the other hand, the acid underwent heterocyclization upon the treatment with 2-aminopyridine, o-phenylenediamine, aryldithiocarbamates and thiourea derivatives to give the corresponding pyridopyrimidine, quinoxalone, 2-thioxo-1,3-thiazole and 4-hydroxy-1,3-thiazole, respectively. The thiazolopyridazine derivatives were obtained from the reaction of 4-hydroxy-1,3-thiazole with hydrazine and phenylhydrazine, respectively. The behaviour of the 4-hydroxy-1,3-thiazole toward acetic anhydride and bromine was also studied. The proposed structures of the products were based on microanalytical and spectroscopic data. Some of the synthesized compounds also exhibited anti-microbial activities.

Different heterocyclic compounds have been synthesized from the reaction of 4-[4-methoxy-3-methylphenyl]-4-oxobutenoic acid with different nucleophiles. Some of the synthesized compounds exhibited antimicrobial activities against different strains of Gram-negative and Gram-positive bacteria.



Similar content being viewed by others
References
Kirchner F K, Bailey J H and Cavallito C J 1949 J. Amer. Chem. Soc. 71 1210
Dal Pozzo A, Acquasaliante M, Donezzeli G, De M aria P and Nicoli C 198 7 J. Med. Chem. 30 1674
Bowden K, Dal Pozzo A and Duah C K 1990 J. Chem. Res. Synopses 2801
Teichert A, Lubken T, Schmidt J, Porzel A, Arnold N and Wessjohann L 2005 Z. Naturforsch. A. 60 25
Bianchi M, Butti A, Christidis Y, Perronnet J, Barzaghi F, Cesana R and Nencioni A 1988 Eur. J. Med. Chem. 23 45
Giordani A, Pevarello P, Speciale C and Varasi M 2000 US Pat. 6 048 896
Juranić Z, Stevović Lj, Drakulić B, Stanojković T, Radulović S and Juranić I 1999 J. Serb. Chem. Soc. 64 505
Drakulić B J, Stanojković T P, Zizak Z S and Dabović M M 2011 Eur. J. Med. Chem. 46 3265
Kolos N N and Beryozkina T 2005 Chem. of Heterocyclic Comp. 41 (11) 1432
Jakubec P, Berkeš D, Šiška R, Gardianová M and Považanec F 2006 Tetrahedron: Asymmetry 17 1629
Kolos N N, Kovalenko L U and Borovskoy V A 2011 Chem. of Heterocyclic Comp. 47 (8) 983
El-Hashash M A, El-Sawy A A, Eissa A M F and Sallam M S 2009 Journal of the Korean Chemical Society 53 (3) 308
Papa D, Schwenk E, Villani F and Klingsberg E 1948 J. Am. Chem. Soc. 70 3356
Soliman M H A and El-Sakka S S 2010 Afinidad 548 316
Cooper K E and Kavanagh E E 1972 In Analytical Microbiology Vol. 2 (New York: Academic Press) p 13
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
EL-SAKKA, S.S., SOLIMAN, M.H. & SAFWAT ABDULLAH, R. Behaviour of 4-[4-methoxy-3-methylphenyl]-4-oxobutenoic acid towards nitrogen-containing nucleophiles. J Chem Sci 126, 1883–1891 (2014). https://doi.org/10.1007/s12039-014-0740-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0740-7