Abstract
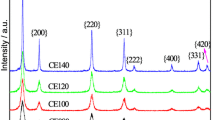
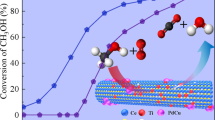
We report here versatile methods to engineer the microstructure and understand the fundamental physicochemical properties of CeO2 to improve its catalytic viability for practical applications. In this context, different morphologies of CeO2 are synthesized using tailored homogeneous precipitation methods and characterized by XRD, BET, SEM and TPR methods. The shuttle-shaped CeO2 prepared under hydrothermal condition shows higher surface area and low-temperature reducibility. The 0.5 wt% Pt-impregnated shuttle-shaped CeO2 shows lower-temperature CO oxidation behaviour as compared to its bulk-like CeO2 (with 0.5 wt% Pt) counterpart, synthesized by conventional-reflux method. Further, nanorod morphology of CeO2 prepared with Cl− as counter ion shows lower-temperature oxidation of soot as compared to the mesoflower morphology of CeO2, prepared with NO\(_{3}^{-}\) as counter ion in the reaction medium. Further, linear sweep voltammetry, chronopotentiometry and CO-stripping voltammetry studies are performed to evaluate the promoting activity of CeO 2 to Pt/C for ethanol electro-oxidation reaction in acidic media. Results show that CeO2 provides active triple-phase-interfacial sites for suitable adsorption of OH species which effectively oxidize the COads on Pt/C. The results presented here are significant in the context of understanding the physicochemical fine prints of CeO2 and CeO2 based hetero-nanocomposites for their suitability to important catalytic and energy-related applications.

CeO2 with engineered microstructure promotes CO oxidation, soot oxidation and alcohol electro-oxidation for energy and environmental applications












Similar content being viewed by others
References
Setten B A A L, Makkee M and Moulijn J A 2001 Catal. Rev. Sci. Eng. 43 489
Freund H, Meijer G, Scheffler M, Schlögl R and Wolf M 2011 Angew. Chem. Int. Ed. 50 10064
Royer S and Duprez D 2011 ChemCatChem 3 24
Herzing A A, Kiely C J, Carley A F, Landon P and Hutchings G J 2008 Science 321 1331
Cargnello M, Wieder N L, Montini T, Gorte R J and Fornasiero P 2010 J. Am. Chem. Soc. 132 1402
Bokhimi X, Zanella R and Angeles-Chavez C 2010 J. Phys. Chem. C114 14101
Bera P, Gayen A, Hegde M S, Lalla N P, Spadaro L, Frusteri F and Arena F 2003 J. Phys. Chem. B107 6122
Zhou H -P, Wu H -S, Shen J, Yin A -X, Sun L D and Yan C H 2010 J. Am. Chem. Soc. 132 4998
Meher S K, Cargnello M, Troiani H, Montini T, Ranga Rao G and Fornasiero P 2013 Appl. Catal. B130–131 121
Ranga Rao G, Fornasiero P, Di Monte R, Kašpar J, Vlaic G, Balducci G, Meriani S, Gubitosa G, Cremona A and Graziani M 1996 J. Catal. 162 1
Esch F, Fabris S, Zhou L, Montini T, Africh C, Fornasiero P, Comelli G and Rosei R 2005 Science 309 752
Meher S K and Ranga Rao G 2012 J. Colloid Interface Sci. 373 46
Si R and Flytzani-Stephanopoulos M 2008 Angew. Chem. Int. Ed. 47 2884
Parida K M and Sahu N 2008 J. Mol. Catal. A 287 151
Parida K M, Mohapatra P, Moma J, Jordaan W A and Scurrell M S 2008 J. Mol. Catal. A288 125
Parida K M, Sahu N, Tripathi A K and Kamble V S 2010 Environ. Sci. Technol. 44 4155
Zhou K, Wang X, Sun X, Peng Q and Li Y 2005 J. Catal. 229 206
Setiabudi A, Chen J, Mul G, Makkee M and Moulijn J A 2004 Appl. Catal. B51 9
Krishna K, Bueno-López A, Makkee M and Moulijn J A 2007 Appl. Catal. B75 189
Guzman J, Carrettin S and Corma A 2005 J. Am. Chem. Soc. 127 3286
Carrette L, Friedrich K A and Stimming U 2001 Fuel Cells 1 5
Lamy C, Lima A, LeRhun V, Delime F, Coutanceau C and Léger J -M 2002 J. Power Sources 105 283
Song S, Maragou V and Tsiakaras P 2007 J. Fuel Cell Sci. Technol. 4 203
Cameron D S, Hards G A, Harrison B and Potter R J 1987 Platinum Met. Rev. 31 173
Lamy C, Belgsir E M and Léger J 2001 J. Appl. Electrochem. 31 799
Cuesta A 2011 ChemPhysChem 12 2375
Yuan H, Guo D, Qiu X, Zhu W and Chen L J 2009 J. Power Sources 188 8
Ranga Rao G, Justin P and Meher S K 2011 Catal. Surv. Asia 15 221
Antolini E and Gonzalez E R 2010 Appl. Catal. B96 245
Meher S K and Ranga Rao G 2012 ACS Catal. 22795
Suffredini H B, Tricoli V, Vatistas N and Avaca L A 2006 J. Power Sources 158 124
Justin P and Ranga Rao G 2009 Catal. Today 141 138
Justin P, Charan P H K and Ranga Rao G 2010 Appl. Catal. B100 510
Justin P and Ranga Rao G 2011 Int. J. Hydrogen Energy 36 5875
Meher S K and Ranga Rao G 2013 J. Phys. Chem. C117 4888
Xu C and Shen P K 2004 Chem. Commun. 2238
Chu Y -Y, Wang Z -B, Jiang Z -Z, Gu D -M and Yin G -P 2011 Adv. Mater. 23 3100
Bambagioni V, Bianchini C, Chen Y, Filippi J, Fornasiero P, Innocenti M, Lavacchi A, Marchionni A, Oberhauser W and Vizza F 2012 ChemSusChem 5 1266
Meher S K, Justin P and Ranga Rao G 2011 Nanoscale 3 683
Ranga Rao G 1999 Bull. Mater. Sci. 22 89
Allian A D, Takanabe K, Fujdala K L, Hao X, Truex T J, Cai J, Buda C, Neurock M and Iglesia E 2011 J. Am. Chem. Soc. 133 4498
Wang Z, Wang Q, Liao Y, Shen G, Gong X, Han N, Liu H and Chen Y 2011 Chem. Phys. Chem. 12 2763
Issa M, Petit C, Mahzoul H, Aboukaïs A and Brilhac J -F 2009 Top. Catal. 52 2063
Liu J, Zhao Z, Liang P, Xu C, Duan A, Jiang G, Lin W and Wachs I E 2008 Catal. Lett. 120 148
Machida M, Murata Y, Kishikawa K, Zhang D and Ikeue K 2008 Chem. Mater. 20 4489
Anumol E A, Kundu P, Deshpande P A, Madras G and Ravishankar N 2011 ACS Nano 5 8049
Hu C -C and Liu K -Y 1999 Electrochim. Acta 44 2727
Léger J -M, Rousseau S, Coutanceau C, Hahn F and Lamy C 2005 Electrochim. Acta 50 5118
Acknowledgements
We thank Ministry of New and Renewable Energy, New Delhi, for providing the CHI 7081C electrochemical workstation to carry out the electrochemical measurements and the SERC Division of DST, Ministry of Science and Technology, New Delhi, for providing powder XRD and BET facilities (under FIST Schemes). We also thank Prof. Paolo Fornasiero, Department of Chemistry, University of Trieste, Italy for CO oxidation measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MEHER, S.K., RAO, G.R. Novel nanostructured CeO 2 as efficient catalyst for energy and environmental applications. J Chem Sci 126, 361–372 (2014). https://doi.org/10.1007/s12039-014-0570-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-014-0570-7