Abstract
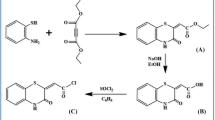
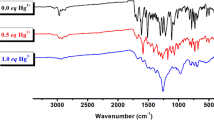
An amido-linked rhodamine conjugate of calix[4]arene, L has been synthesized and characterized. Metal ion recognition properties of L have been studied by emission and absorption techniques with 14 different metal ions including the transition ones. Results show that, L exhibits ratiometric emission intensity towards Hg2+, Fe2+, Fe3+, Cu2+, Pb2+ and Zn2+. Composition of the complex formed in the solution has been found to be 1:2 (L:Mn + ), based on the Job’s plot. The L can also act as a chemosensor for Hg2+ through naked eye detection. Fluorescence quenching observed at 485 nm follows an order, Hg2+ > > Fe3+ ~ Cu2+ > Zn2+ > Pb2+ > Ca2+, while the enhancement observed at 580 nm follows, Hg2+ > > Fe2+ ~ Pb2+ > Zn2+. Mode of interaction of Mn + with L is by the ring opening of spirolactam moiety.

An amido-linked rhodamine conjugate of calix[4]arene, L has been synthesized and characterized and was shown to exhibit ratiometric emission towards Hg2+, Fe2+, Fe3+, Cu2+, Pb2+ and Zn2+. L can act as a chemosensor for Hg2+ by showing a colour change and by forming a 1:2 L:M n+ complex while opening the spirolactam moiety.











Similar content being viewed by others
References
(a) Kubin R F and Fletcher A N 1982 J. Lumin. 27 455; (b) Kojima H, Hirotani M, Nakatsubo N, Kikuchi K, Urano Y, Higuchi T, Hirata Y and Nagano T 2001 Anal. Chem. 73 1967; (c) Chen X, Nam S–W, Jou M J, Kim Y, Sung-Jin Kim S–J, Park S and Yoon J 2008 Org. Lett. 10 5235; (d) Yang H, Zhou Z, Huang K, Yu M, Li F, Yi T and Huang C 2007 Org. Lett. 9 4729
(a) Lohar S, Banerjee A, Sahana A, Banik A, Mukhopadhyay S K and Das D 2013 Anal. Methods 5 442; (b) Wang Z, Wu D, Wu G, Yang N and Wu A 2013 J. Hazard. Mater. 244 621; (c) Chen X, Pradhan T, Wang F, Kim J S and Yoon J 2012 Chem. Rev. 112 1910; (d) Jun M E and Ahn K H 2010 Org. Lett. 12 2790
Shiraishi Y, Sumiya S, Kohno Y and Hirai T 2008 J. Org. Chem. 73 8571
(a) Dujols V, Ford F and Czarnik A W 1997 J. Am. Chem. Soc. 119 7386; (b) Chen X, Jou M J, Lee H, Kou S, Lim J, Nam S-W, Park S, Kim K-M and Yoon J 2009 Sensors Actuators B 137 597
Kwon J Y, Jang Y J, Lee Y J, Kim K M, Seo M S, Nam W and Yoon J 2005 J. Am. Chem. Soc. 127 10107
(a) Othman A B, Lee J W, Wu J-S, Kim J S, Abidi R, Thuery P, Strub J M, Dorsselaer A V and Vicens J 2007 J. Org. Chem. 72 7634; (b) Lee Y H, Lee M H, Zhang J F and Kim J S 2010 J. Org. Chem. 75 7159
Zheng X Y, Zhang W J, Mu L, Zeng X, Xue S F, Tao Z and Yamatob T 2010 J. Incl. Phenom. Macrocycl. Chem. 68 139
Collins E M, McKervey M A, Madigan E, Moran M B, Owens M, Ferguson G and Harris S J 1991 J. Chem. Soc. Perkin Trans. 1 3137
Soh J H, Swamy K M K, Kim S K, Kim S, Lee S-H and Yoon J 2007 Tetrahedron Lett. 48 5966
(a) Kim H N, Lee M H, Kim H J, Kim J S and Yoon J 2008 Chem. Soc. Rev. 37 1465; (b) Li C-Y, Zhou Y, Li Y-F, Kong X–F, Zou C–X and Weng C 2013 Anal. Chim. Acta. 774 79
(a) Benesi H A and Hildebrand J H 1949 J. Am. Chem. Soc. 71 2703; (b) Mukhopadhyay M, Banerjee D, Koll A, Mandal A, Filarowski A, Fitzmaurice D, Das R and Mukherjee S 2005 J. Photochem. Photobiol. A: Chem. 125 94
Acknowledgements
CPR gratefully acknowledges the financial support from the Department of Science and Technology (DST), the Council of Scientific and Industrial Research (CSIR), New Delhi and Department of Atomic Energy (DAE) Board of Research in Nuclear Sciences (BRNS). JPC gratefully acknowledges CSIR for his research fellowships. JD gratefully acknowledges Defense Research and Development Laboratory (DRDL) for allowing him to register for Ph.D. programme at Indian Institute of Technology Bombay. We thank Dr. J K Khedkar for extending help during the revision process.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
CHINTA, J.P., DESSINGOU, J. & RAO, C.P. Synthesis, characterization and ion recognition studies of lower rim 1,3-di{rhodamine} conjugate of calix[4]arene. J Chem Sci 125, 1455–1461 (2013). https://doi.org/10.1007/s12039-013-0500-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0500-0