Abstract
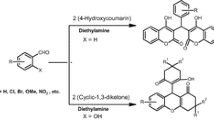
A simple and efficient synthesis of E-9-aryl-5-arylidene-1-oxo-1,2,3,4,5,6,7,8-octahydroxanthenes and their lower analogues has been developed by amberlyst–15 catalysed cyclocondensation of E,E-α,α′-diarylidenecyclohexanones and E,E-α,α′-diarylidenecyclopentanones, respectively, with cyclohexan-1,3-diones. The products were obtained in moderate to good yield and their structures were confirmed from analytical and spectral data.

A simple and efficient synthesis of E-9-aryl-5-arylidene-1-oxo-1,2,3,4,5,6,7,8-octahydroxanthenes and their lower analogues has been developed by amberlyst-15 catalysed cyclocondensation of E,E-α,α′-diarylidenecyclohexanones and E,E-α,α′-diarylidenecyclopentanones, respectively, with cyclohexan-1,3-diones in anhydrous acetonitrile.


Similar content being viewed by others
References
Lambert R W, Martin J A, Merrett J H, Parkes K E B and Thomas G J 1997 PCT Int. Appl. WO9706178, 1997 Chem. Abstr. 126 p212377y
Hideo T 1981 Tokkyo Koho Jpn. JP 56005480, 1981 Chem. Abstr. 95 80922b
Poupelin J P, Saint-Rut G, Fussard-Blanpin O, Narcisse G, Uchida-Ernouf G and Lakroix R 1978 Eur. J. Med. Chem. 13 67
Llama E F, Campo C B, Campo M and Anadon M 1989 Eur. J. Med. Chem. 24 391
Chibale K, Visser M, Schalkwyk D V, Smith P J, Saravanamuthu A and Fairlamb A H 2003 Tetrahedron 59 2289
(a) Cingolant G M and Pigini M 1988 J. Med. Chem. 12 531; (b) Hatakeyma S, Ochi N, Numata H and Takano S 1988 J. Chem. Soc., Chem. Commun. 24 1202
(a) Arnone A, Merlini L and Nasini G 1972 Tetrahedron Lett. 13 3503; (b) Ravindranath B and Sheshadri T R 1973 Phytochemistry 12 2781; (c) Kinjo J, Uemura H, Nohara T, Yamashita N, Marubayashi N and Yoshihira K 1995 Tetrahedron Lett. 36 5599
Banerjee A and Mukherjee A K 1981 Stain Technol. 56 83
(a) Bekaert A, Andrieux J and Plat M 1992 Tetrahedron Lett. 33 2805; (b) Sarma R J and Baruah J B 2005 Dyes Pigm. 64 91; (c) Buehler C A, Cooper D E and Scrudder E O 1943 J. Org. Chem. 8 316; (d) Knight C G and Stephens T 1989 Biochem. J. 258 683
(a) Menchen S M, Benson S C, Lam J Y L, Zhen W, Sun D, Rosenblum B B, Khan S H and Taing M 2003 US Patent, US6583168, 2003 Chem. Abstr. 139 p54287f; (b) Sirkecioglu O, Tulinli N and Akar A, 1995 J. Chem. Res. (S) 1995 502
(a) Ion R M, Frackowiak D, Planner A and Wiktorowicz K 1998 Acta Biochim. Pol. 45 833; (b) Ion R M 1997 Prog. Catal. 6 55
(a) Hamada Y, Matsuura F, Oku M, Hatano K and Shioiri T 1997 Tetrahedron Lett. 38 8961; (b) Hillebrand S, Bruckmann J, Kruger C and Haenel M W 1995 Tetrahedron Lett. 36 75; (c) Malaise G, Barloy L and Osborn J A 2001 Tetrahedron Lett. 42 7417
Few recent references: (a) Rashedian F, Saberi D and Niknam K 2010 J. Chin. Chem. Soc. 57 99; (b) Oskooie H A, Tahershamsi L, Heravi M M and Baghernejad B 2010 E-J. Chem. 7 717; (c) Mahdavinia G H, Ghanbari M M, Sepehrian H and Kooti F 2010 J. Iranian Chem. Res. 3 117; (d) Ali J, Majid M H and Fatemeh F B 2011 E-J. Chem. 8 910; (e) Pramanik A and Bhar S 2012 Catal. Commun. 20 17
(a) Jiao C, Jian S and Chao-guo Y 2011 Chem. Res. Chin. Univ. 27 49; (b) Lasemi Z and Mehrasbi E, 1st National Iranian New Chemistry Congress 5–6 May, 2011, Shiraz
Wang J, Han G, Wu X, Yin J and Zhao Y 2003 Chin. J. Org. Chem. 23 827
Tahmassebi D, Bryson, J A and Binz S I 2011 Synth. Commun. 41 2701
(a) Pal R, Mandal T K, Guha C and Mallik A K 2011 J. Indian Chem. Soc. 88 711; (b) Mandal T K, Pal R, Mondal R and Mallik A K 2011 E- J. Chem. 8 863
(a) Mallik A K, Pal R and Mandal T K 2007 Indian J. Chem. 46B 2056; (b) Pal R, Mandal T K and Mallik A K 2009 J. Indian Chem. Soc. 86 402; (c) Pal R, Mandal T K, Samanta S and Mallik A K 2010 J. Indian Chem. Soc. 87 711
Acknowledgements
Financial assistance from the UGC-CAS and DST-PURSE programs, Department of Chemistry is gratefully acknowledged. The authors also acknowledge the DST-FIST program to the Department of Chemistry, Jadavpur University and Prof. D Mal, Department of Chemistry, Indian Institute of Technology (IIT), Kharagpur for providing the NMR spectral data. SS and AD are thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi and RM to the University Grants Commission (UGC), New Delhi for their Research Fellowships.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SAMANTA, S., GUPTA, A.D., MONDAL, R. et al. A simple synthesis of E-9-aryl-5-arylidene-1-oxo-1,2,3,4,5,6,7, 8-octahydroxanthenes and their lower analogues from E,E-α,α′-diarylidenecycloalkanones. J Chem Sci 125, 737–743 (2013). https://doi.org/10.1007/s12039-013-0442-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0442-6