Abstract
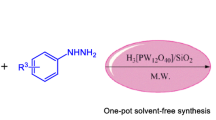
The derivative of triazolo/benzimidazoquinazolinones is prepared via silica-promoted solvent-free method using microwave irradiation with an excellent yield. The newly synthesized compounds were characterized by various techniques like IR, NMR and Mass spectroscopy. The compound with 1a was crystallized and analysed by single crystal X-ray diffraction studies.

An efficient microwave-assisted silica-promoted, solvent-free synthesis of trizaolo/benzimidazoquinazolinones (1a–2h) with an excellent yield are reported and component 1a is crystallized and analysed by single crystal X-ray diffraction studies.



Similar content being viewed by others
References
David J C, Declan C, Timothy P S and Patrick J G 2005 Tetrahedron 61 10153
Liu K C and Hu M K 1986 Arch. Pharm. 319 188
Alagarsamy V and Pathak U S 2007 Bioorg. Med. Chem. 51 3457
Alagarsamy V 2004 Pharmazie 59 753
Alagarsamy V, Murugananthan G and Venkateshperumal R 2003 Biol. Pharm. Bull. 26 1711
Hour M J, Huang L J, Kuo S C, Xia Y, Bastow K, Nakanishi Y, Hamel E and Lee K H 2000 J. Med. Chem. 43 4479
Xia Y, Yang Z Y, Hour M J, Kuo S C, Xia P, Bastow K F, Nakanishi Y, Nampoothiri P, Hackl T, Hamel E and Lee K H 2001 Bioorg. Med. Chem. Lett. 11 1193
Alagarsamy V, Revathi R, Meena S, Ramaseshu K V, Rajasekaran S and De C E 2004 Indian J. Pharm. Sci. 66 459
Lipson V V, Desenko S M, Shirobokova M G and Borodina V V 2003 Chem. Het. Comp. 39 1213
Aboul-Fetouh E M, Ashraf A A, Hassan H F and Eman A B 2007 Beilstein J. Org. Chem. 3 11, doi:10.1186/1860-5397-3-11
(a) Heravi M M, Ranjbar L, Derikvand F, Alimadadi B and Oskooie H A 2008 Mol. Divers. 12 181; (b) Heravi M M, Ranjbar L, Derikvand F and Ranjbar L 2010 Synth. Commun. 40 677
Zirani G M, Alireza B, Zeinab A and Negar L 2011 Arabian J. Chem., doi:10.1016/j.arabjc.2011.06.020
(a) Oliver K 2004 Angew. Chem. Int. Ed. 43 6250; (b) Oliver K 2005 Microwaves in organic and medicinal chemistry, Alexander Stadler, Weinheim: Wiley-vch Verlag GmbH & Co. KGaA
Oliver K and Doris D 2006 Nature Reviews Drug Discovery 5 51
Tanaka K and Toda F 2000 Chem. Rev. 100 1025
(a) Alexander Y U and Yuri L K 2000 Tetrahedron Letters 41 5031; (b) Kidwai M, Misra P, Bhushan K R, Saxena R K and Singh M 2000 Monatsh. Chem. 131 937
Veena Y and Nishant Y 2011 Archives Applied Science Reseasrch 3(1) 139
(a) Oxford Diffraction 2009, Crys alis PRO. Oxford Diffraction Ltd, Yarnton, England; (b) Sheldrick G M 2008 Acta. Cryst. A64 112; (c) Farrugia L J 1997 J. Appl. Crystallogr. 30 565; (d) Sheldrick G M 1997 SHELXS-97 and SHELXL-97, Program of Crystal Structure Refinement (University of Göttingen, Germany)
Details of X-ray single crystal structure determination of 1a was deposited at CCDC (deposition No. CCDC 875381)
6,6-dimethyl-9-phenyl-5,6,7,9-tetrahydro[1,2,4]triazolo[5,1-b]quinazolin-8(4H)-one(1a). Light yellow solid (90%), mp = 248–250°C. 1H-NMR (DMSO-d6, 300 MHz): δ, ppm, 0.95 (s, 3H),1.03 (s, 3H), 2.07 (d, J = 16 Hz, 1H), 2.21 (d, J = 16 Hz, 1H), 2.49 (m, 2H), 5.90 (br, 1H), 7.16–7.27 (m, 5H), 7.67 (s, 1H), 11.02 (s, 1H). IR (KBr): ν: 1650, 1587, 1546, 1473, 1415 cm − 1; LC-MS m/z: C17H18N4O calculated 294.35 found 295.2 [M + H]
Acknowledgements
The authors thank the University Grants Commission (UGC-DRS), (UGC-DSA) and the Department of Science and Technology (DST)-FIST for the Instrumental facility at our Department Premises, Bangalore University Internal Research Fund (BUIRF) Bangalore University for financial support, and Indian Institute of Science (IISc), Bangalore for Spectral studies. KVJ thanks SC/ST cell of Bangalore University for research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
KRISHNAMURTHY, G., JAGANNATH, K.V. Microwave-assisted silica-promoted solvent-free synthesis of triazoloquinazolinone and benzimidazoquinazolinones. J Chem Sci 125, 807–811 (2013). https://doi.org/10.1007/s12039-013-0398-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-013-0398-6