Abstract
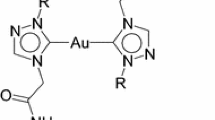
Two silver(I) complexes {[1-R-4-(N-t-butylacetamido)-1,2,4-triazol-5-ylidene]2Ag} + Cl − [R = Et (1b), i-Pr (2b)] of N/O-functionalized N-heterocyclic carbenes derived from 1,2,4-triazoles are reported. The silver complexes, 1b and 2b, have been synthesized from the reaction of the N/O-functionalized triazolium chloride salts namely, 1-R-4-(N-t-butylacetamido)-1,2,4-triazolium chloride [R = Et (1a), i-Pr (2a)] by treatment with Ag2O in 53–56% yield. The 1,2,4-triazolium chloride salts 1a and 2a were prepared by the alkylation reaction of 1-R-1,2,4-triazole (R = Et, i-Pr) with N-t-butyl-2-chloro acetamide in 47–63% yield. The molecular structures of the silver(I) complexes, 1b and 2b, have been determined by X-ray diffraction studies. The density functional theory studies on the silver 1b and 2b complexes suggest that the 1,2,4-triazole derived N-heterocyclic carbenes to be strong σ −donating ligands similar to the now much recognized imidazole-based N-heterocyclic carbenes. The reactivity studies with (SMe2)AuCl and (SMe2)CuBr indicated the silver complexes, 1b and 2b, to be good transmetallating agents.

A rare series of silver(I) complexes of 1,2,4-triazole-based N/O-functionalized N-heterocyclic carbenes were designed with their intended use as transmetallating agents.
Similar content being viewed by others
References
(a) Pugh D and Danopoulos A A 2007 Coord. Chem. Rev. 251 610; (b) Crudden C M and Allen D P 2004 Coord. Chem. Rev. 248 2247; (c) Peris E and Crabtree R H 2004 Coord. Chem. Rev. 248 2239; (d) Herrmann W A 2002 Angew. Chem. Int. Ed. 41 1290
Yen S K, Koh L L, Hahn F E, Huynh H V and Hor T S A 2006 Organometallics 25 5105
(a) Zanardi A, Mata J A and Peris E 2009 Organometallics 28 4335; (b) Ros A, Alcarazo M, Iglesias-Sigüenza J, Díez E, Álvarez E, Fernández R and Lassaletta J M 2008 Organometallics 27 4555; (c) Gnanamgari D, Moores A, Rajaseelan E and Crabtree R H 2007 Organometallics 26 1226
Mathew P, Neels A and Albrecht M 2008 J. Am. Chem. Soc. 130 13534
Schütz J, Herdtweck E and Herrmann W A 2004 Organometallics 23 6084
(a) Samantaray M K, Pang K, Shaikh M M and Ghosh P 2008 Dalton Trans. 4893; (b) Samantaray M K, Pang K, Shaikh M M and Ghosh P 2008 Inorg. Chem. 47 4153; (c) Ray L, Shaikh M M and Ghosh P 2008 Inorg. Chem. 47 230; (d) Samantaray M K, Roy D, Patra A, Stephen R, Saikh M, Sunoj R B and Ghosh P 2006 J. Organomet. Chem. 691 3797
(a) John A and Ghosh P 2010 Dalton Trans. 39 7183; (b) Ray S, Mohan R, Singh J K, Samantaray M K, Shaikh M M, Panda D and Ghosh P 2007 J. Am. Chem. Soc. 129 15042; (c) Ray S, Asthana J, Tanski J M, Shaikh M M, Panda D and Ghosh P 2009 J. Organomet. Chem. 694 2328
(a) Kumar S, Shaikh M M and Ghosh P 2009 J. Organomet. Chem. 694 4162; (b) Ray L, Shaikh M M, and Ghosh P 2007 Organometallics 26 958; (c) Ray L, Shaikh M M, Ghosh P 2007 Dalton Trans. 4546
Dash C, Shaikh M M and Ghosh P 2009 Eur. J. Inorg. Chem. 1608
(a) John A, Shaikh M M and Ghosh P 2009 Dalton Trans. 10581; (b) Samantaray M K; Shaikh M M and Ghosh P 2009 J. Organomet. Chem. 694 3477; (b) Ray L, Barman S, Shaikh M M and Ghosh P 2008 Chem. Eur. J. 14 6646
(a) Samantaray M K, Shaikh M M and Ghosh P 2009 Organometallics 28 2267; (b) Ray S, Shaikh M M and Ghosh P 2009 Eur. J. Inorg. Chem. 1932
(a) Ray L, Katiyar V, Barman S; Raihan M J, Nanavati H, Shaikh M M and Ghosh P 2007 J. Organomet. Chem. 692 4259; (b) Samantaray M K, Katiyar V, Pang K, Nanavati H and Ghosh P 2007 J. Organomet. Chem. 692 1672; (c) Ray L, Katiyar V, Raihan M J, Nanavati H, Shaikh M M and Ghosh P 2006 Eur. J. Inorg. Chem. 3724; (d) Samantaray M K, Katiyar V, Roy D, Pang K, Nanavati H, Stephen R, Sunoj R B and Ghosh P 2006 Eur. J. Inorg. Chem. 2975
Dallacker F and Minn K 1986 Chemiker-Zeitung 110 101
Bulger P G, Cottrell I F, Cowden C J, Davies A J and Dolling U-H 2000 Tetrahedron Lett. 41 1297
Brandys M-C, Jennings M C and Puddephatt R J 2000 J. Chem. Soc., Dalton Trans. 4601
Kiyotsuka Y, Acharya H P, Katayama Y, Hyodo T and Kobayashi Y 2008 Org. Lett. 10 1719
(a) Sheldrick G M 1997 SHELXL-97, Program for refinement of crystal structures (Germany: University of Gottingen); (b) Sheldrick G M 1997 SHELXS-97, Structure solving program (Germany: University of Gottingen)
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, Montgomery J A Jr, Vreven T, Kudin K N, Burant J C, Millam J M, Iyengar S S, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson G A, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J E, Hratchian H P, Cross J B, Adamo C, Jaramillo J, Gomperts R, Stratmann R E, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski J W, Ayala P Y, Morokuma K, Voth G A, Salvador P, Dannenberg J J, Zakrzewski V G, Dapprich S, Daniels A D, Strain M C, Farkas O, Malick D K, Rabuck A D, Raghavachari K, Foresman J B, Ortiz J V, Cui Q, Baboul A G, Clifford S, Cioslowski J, Stefanov B B, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin R L, Fox D J, Keith T, Al-Laham M A, Peng C Y, Nanayakkara A, Challacombe M, Gill P M W, Johnson B, Chen W, Wong M W, Gonzalez C and Pople J A 2004 GAUSSIAN 03: Gaussian 03, Revision C.02, Gaussian, Inc., (Wallingford, CT)
Becke A D 1988 Phys. Rev. A 38 3098
(a) Alkauskas A, Baratoff A and Bruder C 2004 J. Phys. Chem. A 108 6863; (b) Andrae D, Häussermann U, Dolg M, Stoll H and Preuß H 1990 Theor. Chim. Acta 77 123
Hehre W J, Ditchfield R and Pople J A 1972 J. Chem. Phys. 56 2257
Reed A E, Curtiss L A and Wienhold F 1988 Chem. Rev. 88 899
Dapprich S and Frenking G 1995 J. Phys. Chem. 99 9352
(a) Vyboishchikov S F and Frenking G 1998 Chem. Eur. J. 8 4 1439; (b) Frenking G and Pidun U 1997 J. Chem. Soc., Dalton Trans. 1653
Gorelsky S I 1997 AOMix: Program for molecular orbital analysis. (Toronto, Canada: York University); http://www.sg-chem.net/ (accessed June 27, 2009)
Gorelsky S I, Ghosh S and Solomon E I 2006 J. Am. Chem. Soc. 128 278
Pauling L 1960 The nature of the chemical bond (Ithaca, NY: Cornell University Press) 3rd edn, p 224–228, 256–258
Bondi A 1964 J. Phys. Chem. 68 441
Guerret O, Solé S, Gornitzka H, Teichert M, Trinquier G and Bertrand G 1997 J. Am. Chem. Soc. 119 6668
Guerret O, Solé S, Gornitzka H, Trinquier G and Bertrand G 2000 J. Organomet. Chem. 600 112
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
DASH, C., SHAIKH, M.M. & GHOSH, P. Silver complexes of 1,2,4-triazole derived N-heterocyclic carbenes: Synthesis, structure and reactivity studies. J Chem Sci 123, 97–106 (2011). https://doi.org/10.1007/s12039-011-0105-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-011-0105-4