Abstract
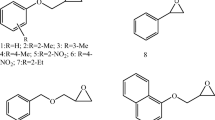
Biocatalytic azidolysis of 9 unsymmetrical epoxides by halohydrin dehalogenase enzyme (HheC) in gas phase and uncatalysed azidolysis of the same epoxides in gas phase and in aqueous solution have been modelled at DFT level. Aliphatic epoxides (1–6) and aromatic epoxides (9) undergo β cleavage while styrene oxide (7) and p-nitro styrene (8) oxide prefer α cleavage in the gas phase. Inclusion of aqueous solvation effect via Polarizable Continuum Model (PCM) increases the activation barrier and makes the reaction endothermic due to extensive solvation of azide anion and oxido anionic products, but does not alter the regioselectivity. Halohydrin dehalogenase from Agrobacterium radiobactor AD1 catalyses (E1–E9) ring opening of all these epoxides by azide ion with β selectivity and the reversal of selectivity in epoxide 7 and 8 is notable. These reactions follow, in both enzymatic and non-enzymatic environment, S N 2 mechanism. Calculations while agreeing totally with experimental results offer better insights on the factors determining the regioselectivity and particularly the role of enzyme. Active site model and crystal structure data reveal that the Tyr145 and Ser132 form weak hydrogen bonds with epoxide oxygen lone pair and form reactant enzyme complex (REC). The enzyme complex activates the epoxide ring towards azidolysis. The NBO deletion and second order perturbation analyses clearly bring out the role of catalytic duo Tyr145 and Ser132 and particularly shed light on the dominant contribution of Tyr145 in selectively activating C β –O bond. The present results indicate that Arg149 or other residues in the pocket do not seem to have any significant effect on the reaction.

The bio-catalysed and uncatalysed ring opening of various epoxides by azide anion have been modelled at B3LYP/6-31+g(2d, p) level. Nine epoxides that include six aliphatic (1–6) and three aromatic (7–9) epoxides have been used for the study and experimental reports where available have been compared.
Similar content being viewed by others
References
(a) Archer I V J 1997 Tetrahedron 53 15617 (b) Nakamura T, Nagasawa T, Yu F, Watanabe I and Yamada H 1994 Appl. Environ. Microbiol. 60 1297 (c) Castro C E and Bartnicki E W 1968 Biochemistry 7 3213 (d) Assis H M S, Sallis P J, Bull A T and Hardman D J 1998 Enzyme Microb. Technol. 22 568 (e) Van den Wijngaard A J, Reuvekamp P T W and Janssen D B 1991 J. Bacteriol. 173 124
Orru R A V and Faber K 1999 Curr. Opin. Chem. Biol. 3 16
Patai S (Ed.) 1971 The Chemistry of Azido group. (New York: Wiley)
Jeffrey L S H, Johan E T, Van Hylckama V, Lixia T, Janssan D B and Kellogg R M 2001 Org. Lett. 3 41
Chini M, Crotti P and Macchia F 1990 Tetrahedron Lett. 31 5641
Blumenstein J J, Ukachukwu V C, Mohan R S and Whalen D L 1993 J. Org. Chem. 58 924
(a) Hopmann H K and Fahmi H 2008 Biochemistry 47 4973 (b) Kleiner C M and Schreniner P R 2006 Chem. Commun. 28 4315 (c) Thomsen R and Christensen M H 2006 J. Med. Chem. 49 3315 (d) Hopmann H K and Fahmi H 2008 J. Chem. Theory. Comput. 4 1129
Janssen D B, Majerić-Elenkov M, Hasnaoui G, Hauer B and Lutje Spelberg J H 2006 Biochem. Soc. Trans. 34 291
Lutje Spelberg J H, Tang L, Van Gelder M, Kellogg R M and Janssen D 2002 Tetrahedron Asymmetry. 13 1083
Elenkov M M, Tang L, Hauer B and Janssen D B 2006 Org. Lett. 8 4227
Elenkov M M, Bernhard H and Janssen D B 2006 Adv. Synth. Catal. 348 579
Haak R M, Tarabiono C, Janssen D B, Minnaard A J, De Varies J G and Feringa B L 2007 Org. Biomol. Chem. 5 318
Mladenovic M, Konstantin J, Reinhold J J, Thiel W, Schirmeister T and Engels B 2008 J. Phys. Chem. B. 112 5458
Frisch M J et al. 2003 Gaussian, Inc, Wallingford CT, Gaussian 03, Revision C.02
De Jong R M, Tiesinga J J, Rozeboom H J, Kalk H, Tang L, Janssen D B and Dijkstra P W 2003 EMBO J. 22 4933
(a) Zhang X, DeChancie J, Gunaydin H, Chowdry A B, Clemente F R, Smith A J, Handel T M and Houk K N 2008 J. Org. Chem. 73 889, (b) Claeyssens F, Harvey J N, Mulholland A J, Ranaghan K E, Schütz M, Thiel S, Thiel W and Werner H J 2006 J. Angew. Chem Int. Ed. 45 6856
Laitinen T, Rouvinen J and Peräkylä M J 1998 J. Org. Chem. 63 8157
Tamami B, Iranpoor N and Mahdavi H 2002 Synth. Commun. 32 1251
Elenkov M M, Tang L, Meetsma A, Hauer B and Janssen D B 2008 Org. Lett. 10 2417
De Jone R. M, Jan J, Tiesinga W, Alessandra V, Tang L and Janssen D B 2005 J. Am. Chem. Soc. 127 13338
Hasnaoui G, Jeffrey H, Spelberg L, Erik de Vries, Tang L, Hauer B and Janssen D B 2005 Tetrahedron Asymmetry. 16 1685
Xu W, Xu J H, Pan J, Qing G and Xin-Yan W 2006 Org. Lett. 8 1737
Ammantini D, Francesco F, Piermatti. O, Tortoioli S and Vaccaro L 2002 ARKIVOC. XI 293 and references Cited there in.
Oshima T, Haruyasu A, Kubo E, Miyamoto S and Togaya K 2008 Org. Lett. 10 2413
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
SENTHILNATHAN, D., TAMILMANI, V. & VENUVANALINGAM, P. Biocatalysis of azidolysis of epoxides: Computational evidences on the role of halohydrin dehalogenase (HheC). J Chem Sci 123, 279–290 (2011). https://doi.org/10.1007/s12039-011-0082-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-011-0082-7