Abstract
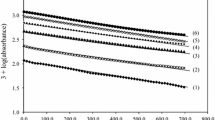
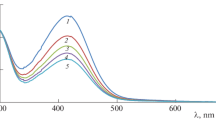
The kinetics of Ru(III) catalysed oxidation of l-lysine by diperiodatoargentate (III) (DPA) in alkaline medium at 298 K and a constant ionic strength of 0·50 mol dm−3 was studied spectrophotometrically. The oxidation products are aldehyde (5-aminopentanal) and Ag (I). The stoichiometry is i.e. [L-lysine]: [DPA] = 1: 1. The reaction is of first order in [Ru(III)] and [DPA] and is less than unit order in both [L-lys] and [alkali]. Addition of periodate had a retarding effect on the reaction. The oxidation reaction in alkaline medium has been shown to proceed via a Ru(III)-L-lysine complex, which further reacts with one molecule of monoperiodatoargentate(III) (MPA) in a rate determining step followed by other fast steps to give the products. The main products were identified by spot test, IR, GC-MS studies. The activation parameters with respect to slow step of the mechanism are computed and discussed and thermodynamic quantities are also determined. The active species of catalyst and oxidant have been identified.
Similar content being viewed by others
References
Lalo D and Mahanti M K 1990 J. Chem. Soc. Dalton Trans. 311
Bal Reddy K, Sethuram B and Navaneeth Rao T 1981 Indian. J. Chem. A20 395
Flodin N W 1997 J. Am. Coll Nutr. 16 7
Sethuram B 2003 Some aspects of electron transfer reactions involving organic molecules (New Delhi: Allied Publishers (P) Ltd) pp 78, 151
Jaiswal P K and Yadav K L 1970 Talanta 17 236
Jaiswal P K 1972 Analyst 1 503
Jayaprakash Rao P, Sethuram B and Navaneeth Rao T 1985 React. Kinet. Catal. Lett. 29 289
Venkata Krishna K and Jayaprakash Rao P 1998 Indian J. Chem. A37 1106 references therein
Kumar A and Kumar P 1999 J. Phys. Org. Chem. 12 79
Kumar A, Vaishali P and Ramamurthy 2000 Int. J. Chem. Kinet. 32 286
Munavalli D S, Chimatadar S A and Nandibewoor S T 2008 Transition. Met. Chem. 33 535
Reddy C S and Vijaykumar T 1995 Indian J. Chem. A34 615
Panigrahi G P and Misro P K 1977 Indian J. Chem. A15 1066
Cohen G L and Atkinson G 1964 Inorg. Chem. 3 1741
Jeffery G H, Bassett J, Mendham J and Denney R C 1996 Vogel’s textbook of quantitative chemical analysis 5th edn (Singapore: Longmans Singapore Publishers Pte Ltd.) pp. 391 and 467
Moelwyn-Hughes E A 1947 Kinetics of reaction in solutions (London: Oxford University Press)
Crouthumel C E, Meek H V, Martin D S and Banus C V 1949 J. Am. Chem. Soc. 71 3031
Crouthamel C E, Hayes A M and Martin D S 1951 J. Am. Chem. Soc. 73 82
Bhattacharya S, Saha B, Datta A and Banerjee P 1988 Coord. Chem. Rev. 47 170
Haines R I and McAuley A 1981 Coord. Chem. Rev. 39 77
Desai S M, Halligudi N N and Nandibewoor S T 2002 Transition Met. Chem. 27 207
Chang R 1981 Physical chemistry with applications to biological systems (New York: McMillan) p. 326
Seregar V C, Veeresh T M and Nandibewoor S T 2007 Polyhedron 26 1731
Weissberger A and Lewis E S (eds) 1974 Investigation of rates and mechanism of reactions in techniques of chemistry (New York: Wiley) 4 421
Farokhi S A and Nandibewoor S T 2003 Tetrahedron 59 7595
Exner O 1964 Coll. Czech. Chem. Commun. 29 1094
Exner O 1972 Coll. Czech. Chem. Commun. 37 1425
Leffler J E 1955 J. Org. Chem. 20 1202
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosamani, R.R., Nandibewoor, S.T. Mechanistic study of ruthenium (III) catalysed oxidation of L-lysine by diperiodatoargentate (III) in aqueous alkaline medium. J Chem Sci 121, 275–281 (2009). https://doi.org/10.1007/s12039-009-0030-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-009-0030-y