Abstract
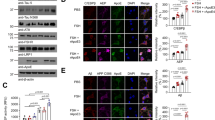
A central hypothesis in the study of Alzheimer’s disease (AD) is the accumulation and aggregation of β-amyloid peptide (Aβ). Recent epidemiological studies suggest that patients with elevated cholesterol and decreased estrogen levels are more susceptible to AD through Aβ accumulation. To test the above hypothesis, we used ovariectomized with diet-induced hypercholesterolemia (OVX) and hypercholesterolemia (HCL) diet alone mouse models. HPLC analysis reveals the presence of beta amyloid in the OVX and HCL mice brain. Congo red staining analysis revealed the extent of amyloid deposition in OVX and hypercholesterolemia mice brain. Overall, Aβ levels were higher in OVX mice than in HCL. Secondly, estrogen receptors α (ERα) were assessed by immunohistochemistry and this suggested that there was a decreased expression of ER α in OVX animals when compared to hypercholesterolemic animals. Aβ was quantified by Western blot and ELISA analysis. Overall, Aβ levels were higher in OVX mice than in HCL mice. Our experimental results suggested that OVX animals were more susceptible to AD with significant increase in Aβ peptide.








Similar content being viewed by others
References
Andrea MR, Nagele RG, Wang HY, Peterson PA and Lee DH 2001 Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer’s disease. Histopathology 38 120–134
Brinton RD, Chen S, Montoya M, Hsieh D and Minaya J 2000 The estrogen replacement therapy of the Women’s Health Initiative promotes the cellular mechanisms of memory and neuronal survival in neurons vulnerable to Alzheimer’s disease. Maturitas 34 35–52
Burns MP, Noble WJ, Olm V, Gaynor K, Casey E, LaFrancois J, Wang L and Duff K 2003 Co-localization of cholesterol, apolipoprotein E and fibrillar Abeta in amyloid plaques. Brain Res. Mol. Brain Res. 110 119–125
Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M and Wolfson L 1988 Clinico pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology 38 1682–1687
De Caterina R and Massaro M 2005 Omega-3 fatty acids and the regulation of expression of endothelial pro-atherogenic and pro-inflammatory genes. J. Membr. Biol. 206 103–116
DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM and Holtzman DM 2001 Peripheral anti-A antibody alters CNS and plasma A clearance and decreases brain A burden in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 98 8850–8855
Duka T, Tasker R and McGowan JF 2000 The effects of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology 149 129–139
Ehehalt R, Keller P, Haass C, Thiele C and Simons K 2003 Amyloidogenic processing of the Alzheimer beta amyloid precursor protein depends on lipids rafts. J. Cell Biol. 160 113–123
Frears ER, Stephens DJ, Walters CE, Davies H and Austen BM 1999 The role of cholesterol in the biosynthesis of beta-amyloid. NeuroReport 10 1699–705
Gandy S and Duff K 2000 Post-menopausal estrogen deprivation and Alzheimer’s disease. Exp. Gerontol. 35 503–511
George AJ, Holsinger RM, McLean CA, Laughton KM, Beyreuther K, Evin G, Masters CL and Li QX 2004 APP intracellular domain is increased and soluble Abeta is reduced with diet-induced hypercholesterolemia in a transgenic mouse model of Alzheimer disease. Neurobiol. Dis. 16 124–132
Goodman Y, Bruce AJ, Cheng B and Mattson MP 1996 Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J. Neurochem. 66 1836–1844
Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME and Buckwalter JG 1994 Estrogen replacement therapy in older women. Comparisons between Alzheimer’s disease cases and no demented control subjects. Arch. Neurol. 51 896–900
Heverin M, Meaney S, Lütjohann D, Diczfalusy U, Wahren J and Björkhem I 2005 Crossing the barrier: net flux of 27-hydroxycholesterol into the human brain. J. Lipid Res. 46 1047–52
Hong-Goka BC and Chang FL 2004 Estrogen receptors alpha and beta in choroid plexus epithelial cells in Alzheimer’s disease. Neurosci. Lett. 360 113–116
Howland DS, Trusko SP, Savage MJ, Reaume AG, Lang DM, Hirsch JD, Maeda N, Siman R, et al. 1998 Modulation of secreted beta-amyloid precursor protein and amyloid beta-peptide in brain by cholesterol. J. Biol. Chem. 273 16576–16582
Jesmin S, Hattori Y, Sakuma I, Liu MY, Mowa CN and Kitabatake A 2003 Estrogen deprivation and replacement modulate cerebral capillary density with vascular expression of angiogenic molecules in middle-aged female rats. J. Cerebr. Blood F. Met. 23 181–189
Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, et al. 1997 A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology 48 1517–1521
Manly JJ, Merchant CA, Jacobs DM, Small SA, Bell K, Ferin M and Mayeux R 2000 Endogenous estrogen levels and Alzheimer’s disease among postmenopausal women. Neurology 54 833–837
Miranda RC, Sohrabji F and Toran Allerand CD 1993 Neuronal colocalization of mRNAs for neurotrophins and their receptors in the developing central nervous system suggests a potential for autocrine interactions. Proc. Natl. Acad. Sci. USA 90 6439–6443
Mufson EJ, Cai WJ, Jaffar S, Chen E, Stebbins G, Sendera T and Kordower JH 1999 Estrogen receptor immunoreactivity within subregions of the rat forebrain: neuronal distribution and association with perikarya containing choline acetyltransferase. Brain Res. 849 253–274
Pappolla MA, Bryant-Thomas TK, Herbert D, Pacheco J, Fabra Garcia M, Manjon M, Girones X, Henry TL, et al. 2003 Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology 61 199–205
Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, et al. 2000 Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 4 321–331
Rena Li, Yong Shen, Li-Bang Yang, Lih-Fen Lue, Finch C and Rogers J 2000 Estrogen enhances uptake of amyloid ß-protein by microglia derived from the human cortex. J. Neurochem. 75 14–47
Sambamurti K, Sevlever D, Koothan T, Refolo LM, Pinnix I, Gandhi S, Onstead L, Younkin L, et al. 1999 Glycosylphosphatidylinositol-anchored proteins play an important role in the biogenesis of the Alzheimer’s amyloid beta-protein. J. Biol. Chem. 274 26810–26814
Shie FS, Jin LW, Cook DG, Leverenz JB and LeBoeuf RC 2002 Diet-induced hypercholesterolemia enhances brain A beta accumulation in transgenic mice. NeuroReport 13 455–459
Sparks DL, Scheff SW, Hunsaker JC 3rd, Liu H, Landers T and, Gross DR 1994 Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp. Neurol. 26 88–94
Toran-Allerand CD, Miranda RC, Bentham WD, Sohrabji F, Brown TJ, Hochberg RB, MacLusky NJ 1992 Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal forebrain. Proc. Natl. Acad. Sci. USA 89 4668–4672
Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, et al. 2001 Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 21 1809–1818
Wolozin B 2004 Cholesterol and the biology of Alzheimer’s disease. Neuron 41 7–410
Zheng H, Xu H, Uljon SN, Gross R, Hardy K, Gaynor J, Lafrancois J, Simpkins J, et al. 2002 Modulation of A (beta) peptides by estrogen in mouse models. J. Neurochem. 80 191–196
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Vidita A Vaidya
MS received 29 September 2011; accepted 09 August 2012
Corresponding editor: Vidita A Vaidya
[Kaliyamurthi V, Thanigavelan V and Rajamanickam GV 2012 Effects of diet-induced hypercholesterolemia on amyloid accumulation in ovariectomized mice. J. Biosci. 37 1–11] DOI 10.1007/s12038-012-9262-y
Rights and permissions
About this article
Cite this article
Kaliyamurthi, V., Thanigavelan, V. & Rajamanickam, G.V. Effects of diet-induced hypercholesterolemia on amyloid accumulation in ovariectomized mice. J Biosci 37 (Suppl 1), 1017–1027 (2012). https://doi.org/10.1007/s12038-012-9262-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-012-9262-y