Abstract
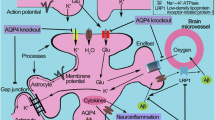
Cognitive impairment refers to notable declines in cognitive abilities including memory, language, and emotional stability leading to the inability to accomplish essential activities of daily living. Astrocytes play an important role in cognitive function, and homeostasis of the astrocyte-neuron lactate shuttle (ANLS) system is essential for maintaining cognitive functions. Aquaporin-4 (AQP-4) is a water channel expressed in astrocytes and has been shown to be associated with various brain disorders, but the direct relationship between learning, memory, and AQP-4 is unclear. We examined the relationship between AQP-4 and cognitive functions related to learning and memory. Mice with genetic deletion of AQP-4 showed significant behavioral and emotional changes including hyperactivity and instability, and impaired cognitive functions such as spatial learning and memory retention. 18 F-FDG PET imaging showed significant metabolic changes in the brains of AQP-4 knockout mice such as reductions in glucose absorption. Such metabolic changes in the brain seemed to be the direct results of changes in the expression of metabolite transporters, as the mRNA levels of multiple glucose and lactate transporters in astrocytes and neurons were significantly decreased in the cortex and hippocampus of AQP-4 knockout mice. Indeed, AQP-4 knockout mice showed significantly higher accumulation of both glucose and lactate in their brains compared with wild-type mice. Our results show that the deficiency of AQP-4 can cause problems in the metabolic function of astrocytes and lead to cognitive impairment, and that the deficiency of AQP4 in astrocyte endfeet can cause abnormalities in the ANLS system.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its Additional information files. Further information is available from the corresponding author (rghree@amc.seoul.kr) upon request.
References
Hu C et al (2017) The prevalence and progression of mild cognitive impairment among clinic and community populations: a systematic review and meta-analysis. Int Psychogeriatr 29(10):1595–1608
Cao Q et al (2020) The prevalence of dementia: a systematic review and Meta-analysis. J Alzheimers Dis 73(3):1157–1166
Bouras C et al (1994) Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of Elderly Patients: a quantitative evaluation of a one-year autopsy Population from a Geriatric Hospital. Cereb Cortex 4(2):138–150
Morris JC et al (1996) Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presymptomatic and very mild Alzheimer’s disease. Neurology 46(3):707–719
Mitchell TW et al (2002) Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer’s disease. Ann Neurol 51(2):182–189
Riley KP, Snowdon DA, Markesbery WR (2002) Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol 51(5):567–577
Schneider JA et al (2009) The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 66(2):200–208
Stephan BC et al (2012) The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Mol Psychiatry 17(11):1056–1076
Gibbs ME, Hutchinson D, Hertz L (2008) Astrocytic involvement in learning and memory consolidation. Neurosci Biobehav Rev 32(5):927–944
Lee HS et al (2014) Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci 111(32):E3343–E3352
Adamsky A, Goshen I (2018) Astrocytes in memory function: pioneering findings and future directions. Neuroscience 370:14–26
Halassa MM et al (2009) Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61(2):213–219
O’Dowd BS et al (1994) Astrocytes implicated in the energizing of intermediate memory processes in neonate chicks. Brain Res Cogn Brain Res 2(2):93–102
Gerlai R et al (1995) Overexpression of a calcium-binding protein, S100 beta, in astrocytes alters synaptic plasticity and impairs spatial learning in transgenic mice. Learn Mem 2(1):26–39
Haydon PG (2001) GLIA: listening and talking to the synapse. Nat Rev Neurosci 2(3):185–193
Anderson CM, Nedergaard M (2003) Astrocyte-mediated control of cerebral microcirculation Trends Neurosci, 26(7): p. 340-4; author reply 344-5
Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26(10):523–530
Rossi DJ, Brady JD, Mohr C (2007) Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci 10(11):1377–1386
Stevens B (2008) Neuron-astrocyte signaling in the development and plasticity of neural circuits. Neurosignals 16(4):278–288
Verkhratsky A, Nedergaard M (2018) Physiol Astroglia Physiol Rev 98(1):239–389
Ullian EM, Christopherson KS, Barres BA (2004) Role for glia in synaptogenesis. Glia 47(3):209–216
Jahanshahi M et al (2008) The effect of spatial learning on the number of astrocytes in the CA3 subfield of the rat hippocampus. Singap Med J 49(5):388–391
Fields RD (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31(7):361–370
Gibbs ME, Hertz L (2008) Inhibition of astrocytic energy metabolism by D-lactate exposure impairs memory. Neurochem Int 52(6):1012–1018
Moraga-Amaro R et al (2014) Role of astrocytes in memory and psychiatric disorders. J Physiol Paris 108(4–6):240–251
Koh W et al (2022) Astrocytes render memory flexible by releasing D-Serine and regulating NMDA receptor tone in the Hippocampus. Biol Psychiatry 91(8):740–752
Agre P, Bonhivers M, Borgnia MJ (1998) The aquaporins, blueprints for cellular plumbing systems. J Biol Chem 273(24):14659–14662
Takata K, Matsuzaki T, Tajika Y (2004) Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem 39(1):1–83
Badaut J et al (2014) Aquaporin and brain diseases. Biochim Biophys Acta 1840(5):1554–1565
Nielsen S et al (1997) Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17(1):171–180
Nagelhus EA, Ottersen OP (2013) Physiological roles of aquaporin-4 in brain. Physiol Rev 93(4):1543–1562
Iliff JJ et al (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4(147):147ra111
Nagelhus EA et al (1999) Immunogold evidence suggests that coupling of K + siphoning and water transport in rat retinal Müller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia 26(1):47–54
Amiry-Moghaddam M, Ottersen OP (2003) The molecular basis of water transport in the brain. Nat Rev Neurosci 4(12):991–1001
Zeng XN et al (2007) Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol Cell Neurosci 34(1):34–39
Benarroch EE (2007) Aquaporin-4, homeostasis, and neurologic disease. Neurology 69(24):2266–2268
Moftakhar P et al (2010) Aquaporin expression in the brains of patients with or without cerebral amyloid angiopathy. J Neuropathol Exp Neurol 69(12):1201–1209
Simpson JE et al (2010) Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol Aging 31(4):578–590
Lee DJ et al (2012) Decreased expression of the glial water channel aquaporin-4 in the intrahippocampal kainic acid model of epileptogenesis. Exp Neurol 235(1):246–255
Tarasoff-Conway JM et al (2015) Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 11(8):457–470
Vella J et al (2015) The central role of aquaporins in the pathophysiology of ischemic stroke. Front Cell Neurosci, 9
Kinboshi M et al (2017) Inhibition of Inwardly rectifying Potassium (Kir) 4.1 channels facilitates brain-derived neurotrophic factor (BDNF) expression in astrocytes. Front Mol Neurosci, 10
O’Donovan SM, Sullivan CR, McCullumsmith RE (2017) The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. npj Schizophrenia 3(1):32
Xing HQ et al (2017) Decrease of aquaporin-4 and excitatory amino acid transporter-2 indicate astrocyte dysfunction for pathogenesis of cortical degeneration in HIV-associated neurocognitive disorders. Neuropathology 37(1):25–34
Garcia-Esparcia P et al (2018) Glutamate transporter GLT1 expression in Alzheimer Disease and Dementia with Lewy Bodies. Front Aging Neurosci 10:122–122
Zoltowska KM et al (2018) Novel interaction between Alzheimer’s disease-related protein presenilin 1 and glutamate transporter 1. Sci Rep 8(1):8718
Liu K et al (2021) Attenuation of cerebral edema facilitates recovery of glymphatic system function after status epilepticus. JCI Insight, 6(17)
Stokum JA et al (2018) SUR1-TRPM4 and AQP4 form a heteromultimeric complex that amplifies ion/water osmotic coupling and drives astrocyte swelling. Glia 66(1):108–125
Pellerin L et al (1998) Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci 20(4–5):291–299
Suzuki A et al (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144(5):810–823
Alberini CM et al (2018) Astrocyte glycogen and lactate: new insights into learning and memory mechanisms. Glia 66(6):1244–1262
Skucas VA et al (2011) Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. J Neurosci 31(17):6392–6397
Woo J et al (2018) Astrocytic water channel aquaporin-4 modulates brain plasticity in both mice and humans: a potential gliogenetic mechanism underlying language-associated learning. Mol Psychiatry 23(4):1021–1030
Kitaura H et al (2009) Activity-dependent glial swelling is impaired in aquaporin-4 knockout mice. Neurosci Res 64(2):208–212
Igarashi H et al (2014) Water influx into cerebrospinal fluid is primarily controlled by aquaporin-4, not by aquaporin-1: 17O JJVCPE MRI study in knockout mice. NeuroReport 25(1):39–43
Ueki S, Suzuki Y, Igarashi H (2021) Retinal Aquaporin-4 and regulation of Water Inflow into the vitreous body. Invest Ophthalmol Vis Sci 62(2):24
Simon P, Dupuis R, Costentin J (1994) Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res 61(1):59–64
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A 91(22):10625–10629
Zimmer ER et al (2017) [(18)F]FDG PET signal is driven by astroglial glutamate transport. Nat Neurosci 20(3):393–395
Small GW et al (2000) Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A 97(11):6037–6042
Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14(6):724–738
Stehberg J et al (2012) Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. Faseb j 26(9):3649–3657
Bazargani N, Attwell D (2016) Astrocyte calcium signaling: the third wave. Nat Neurosci 19(2):182–189
Ostroff LE et al (2014) Synapses lacking astrocyte appear in the amygdala during consolidation of pavlovian threat conditioning. J Comp Neurol 522(9):2152–2163
Han J et al (2012) Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148(5):1039–1050
Orr AG et al (2015) Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci 18(3):423–434
Gibbs ME, Summers RJ (2002) Effects of glucose and 2-deoxyglucose on memory formation in the chick: interaction with beta(3)-adrenoceptor agonists. Neuroscience 114(1):69–79
Gibbs ME, Anderson DG, Hertz L (2006) Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia 54(3):214–222
Yang J et al (2014) Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A 111(33):12228–12233
Gordon GR et al (2008) Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456(7223):745–749
Lauritzen KH et al (2014) Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 24(10):2784–2795
Pierre K et al (2000) Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 100(3):617–627
Lu Y, Christian K, Lu B (2008) BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem 89(3):312–323
Fan Y et al (2013) Aquaporin-4 promotes memory consolidation in Morris water maze. Brain Struct Funct 218(1):39–50
Yang J et al (2013) Chronic ceftriaxone treatment rescues hippocampal memory deficit in AQP4 knockout mice via activation of GLT-1. Neuropharmacology 75:213–222
Li YK et al (2012) Aquaporin-4 deficiency impairs synaptic plasticity and associative fear memory in the lateral amygdala: involvement of downregulation of glutamate transporter-1 expression. Neuropsychopharmacology 37(8):1867–1878
Djukic B et al (2007) Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci 27(42):11354–11365
Barco A et al (2005) Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron 48(1):123–137
Katagiri H, Tanaka K, Manabe T (2001) Requirement of appropriate glutamate concentrations in the synaptic cleft for hippocampal LTP induction. Eur J Neurosci 14(3):547–553
Henneberger C et al (2010) Long-term potentiation depends on release of D-serine from astrocytes. Nature 463(7278):232–236
Shigetomi E et al (2013) TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J Neurosci 33(24):10143–10153
Sultan S et al (2013) D-serine increases adult hippocampal neurogenesis. Front NeuroSci, 7
Mothet JP et al (2006) A critical role for the glial-derived neuromodulator D-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell 5(3):267–274
Acknowledgements
We thank Dr. Eun-Jae Lee for providing the AQP-4 KO mouse. We are also grateful to Dr. Chong Jai Kim for his generous support in equipment and clinical resources. We thank the Metabolomic Core, Positron Emission Tomography core laboratory facilities, the Confocal Microscope Core, and the Laboratory of Animal Research at the ConveRgencemEDIcine research center (CREDIT) at Asan Medical Center for their equipment, services, and expertise.
Funding
This research was supported by a grant from the Korean government Ministry of Science and ICT (MSIT) (2022R1A2C2011941), and 2023IP0037, 2023IP0040 from the Asan Institute for Life sciences, Asan Medical Center (Seoul, Republic of Korea) and KIST Institutional Program(Project No. 2V09540-23-090) to Seungjoo Lee and the Health Fellowship Foundation and the Korean government Ministry of Science and ICT (2022R1C1C2002698) to Hanwool Jeon.
Author information
Authors and Affiliations
Contributions
HC, JHC and HJ performed all experimental procedure and participated to the conceptualization and drafting of the manuscript; JHK, MK, WP, JSL, EL, JSA, JHK, SHH and JEP performed data analysis ; SJK and HJY performed metabolomic analysis on mouse samples; JHJ performed FDG-PET analysis on mouse brain; SL supervised the project and drafted all manuscript. All authors have agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the by the Institutional Animal Care and Use Committee (IACUC) at Asan Medical Center (IACUC no.2020-14-156).
Consent for Publication
None.
Competing Interests
The authors have no potential conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cha, H., Choi, J.H., Jeon, H. et al. Aquaporin-4 Deficiency is Associated with Cognitive Impairment and Alterations in astrocyte-neuron Lactate Shuttle. Mol Neurobiol 60, 6212–6226 (2023). https://doi.org/10.1007/s12035-023-03475-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03475-9