Abstract
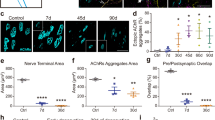
Motor function recovery from injury requires the regeneration of not only muscle fibers, but also the neuromuscular junction—the synapse between motor nerve terminals and muscle fibers. However, unlike muscle regeneration which has been extensively studied, little is known about the molecular mechanisms of NMJ regeneration. Recognizing the critical role of agrin-LRP4-MuSK signaling in NMJ formation and maintenance, we investigated whether increasing MuSK activity promotes NMJ regeneration. To this end, we evaluated the effect of DOK7, a protein that stimulates MuSK, on NMJ regeneration. Reinnervation, AChR cluster density, and endplate area were improved, and fragmentation was reduced in the AAV9-DOK7-GFP-injected muscles compared with muscles injected with AAV9-GFP. These results demonstrated expedited NMJ regeneration associated with increased DOK7 expression and support the hypothesis that increasing agrin signaling benefits motor function recovery after injury. Our findings propose a potentially new therapeutic strategy for functional recovery after muscle and nerve injury, i.e., promoting NMJ regeneration by increasing agrin signaling.






Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available (upon request) in the Mei Lab repository at the Department of Neuroscience, Case Western Reserve University (contact email: ekosco3@yahoo.com).
References
Faroni A, Mobasseri SA, Kingham PJ, Reid AJ (2015) Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev 82–83:160–167
Grinsell D, Keating CP (2014) Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int 2014:698256
Scholz T, Krichevsky A, Sumarto A, Jaffurs D, Wirth GA, Paydar K, Evans GR (2009) Peripheral nerve injuries: an international survey of current treatments and future perspectives. J Reconstr Microsurg 25:339–344
Hartmann JE (2006) Neurology in Operation Iraqi Freedom: risk factors for referral, clinical presentations and incidence of disease. J Neurol Sci 241:83–90
Owens BD, Kragh JF Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB (2008) Combat wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma 64:295–299
Wu H, Xiong WC, Mei L (2010) To build a synapse: signaling pathways in neuromuscular junction assembly. Development 137:1017–1033. https://doi.org/10.1242/dev.038711pmid:20215342
Li L, Xiong WC, Mei L (2018) Neuromuscular Junction Formation, Aging, and Disorders. Annu Rev Physiol 10(80):159–188. https://doi.org/10.1146/annurev-physiol-022516-034255
Sanes JR, Lichtman JW (2001) Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci 2(791):805. https://doi.org/10.1038/35097557pmid:11715056
Carraro U, Catani C, Biral D (1979) Selective maintenance of neurotrophically regulated proteins in denervated rat diaphragm. Exp Neurol 63:468–475
de Castro RA, Andreo JC, Rosa GM Jr, dos Santos NB, Moraes LH, Lauris JR (2007) Fat cell invasion in long-term denervated skeletal muscle. Microsurgery 27(8):664–667. https://doi.org/10.1002/micr.20428 (PMID: 17941108)
McMahan UJ (1990) The agrin hypothesis. Cold Spring Harb Symp Quant Biol 55:407–18
Kim N, Stiegler A, Cameron T, Hallock P, Gomez A, Huang J, Hubbard S, Dustin M et al (2008) Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell 135:334–342
Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L (2008) LRP4 serves as a coreceptor of agrin. Neuron 60:285–297
Zong Y, Zhang B, Gu S, Lee K, Zhou J, Yao G, Figueiredo D, Perry K et al (2012) Structural basis of agrin-LRP4-MuSK signaling. Genes Dev 26(3):247–258
Okada K, Inoue A, Okada M, Murata Y, Kakuta S, Jigami T, Kubo S, Shiraishi H, Eguchi K, Motomura M, Akiyama T, Iwakura Y, Higuchi O, & Yamanashi Y (2006) The Muscle Protein Dok-7 Is Essential for Neuromuscular Synaptogenesis. Science, 312(5781):1802–1805. https://doi.org/10.1126/science.1127142
DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL et al (1996) The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85:501–512. https://doi.org/10.1016/S0092-8674(00)81251-9pmid:8653786
Gautam M, Noakes PG, Mudd J, Nichol M, Chu GC, Sanes JR, Merlie JP (1995) Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature 377:232–236. https://doi.org/10.1038/377232a0pmid:7675108
Weatherbee SD, Anderson KV, Niswander LA (2006) LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development 133:4993–5000. https://doi.org/10.1242/dev.02696pmid:17119023
Pevzner A, Schoser B, Peters K, Cosma NC, Karakatsani A, Schalke B, Melms A, Kröger S (2012) Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis. J Neurol 259(3):427–435. https://doi.org/10.1007/s00415-011-6194-7 (Epub 2011 Aug 5 PMID: 21814823)
Yamashita Rika et al (2021) Anti-MuSK positive myasthenia gravis with anti-Lrp4 and anti-titin antibodies. Internal Med (Tokyo, Japan) 60(1):137–140. https://doi.org/10.2169/internalmedicine.4957-20
Zhang B, Tzartos JS, Belimezi M, Ragheb S, Bealmear B, Lewis RA, Xiong WC, Lisak RP, Tzartos SJ, Mei L (2012) Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch Neurol. 69(4):445–51. https://doi.org/10.1001/archneurol.2011.2393
Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A (2001) Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 7:365–368
Zhang B, Shen C, Bealmear B, Ragheb S, Xiong WC, Lewis RA, Lisak RP, & Mei L (2014). Autoantibodies to Agrin in Myasthenia Gravis Patients. PLoS ONE, 9(3):e91816. https://doi.org/10.1371/journal.pone.0091816
Shen C, Lu Y, Zhang B, Figueiredo D, Bean J, Jung J, Wu H, Barik A et al (2013) Antibodies against low-density lipoprotein receptor-related protein 4 induce myasthenia gravis. J Clin Invest 123(12):5190–202. https://doi.org/10.1172/JCI66039
Feng Z, Lam S, Tenn EMS, Ghosh AS, Cantor S, Zhang W, Yen PF, Chen KS et al (2021) Activation of muscle-specific kinase (MuSK) reduces neuromuscular defects in the delta7 mouse model of spinal muscular atrophy (SMA). Int J Mol Sci 22(15):8015. https://doi.org/10.3390/ijms22158015
Yan M, Liu Z, Fei E, Chen W, Lai X, Luo B, Chen P, Jing H, Pan JX, Rivner MH, Xiong WC, & Mei L (2018) Induction of Anti-agrin Antibodies Causes Myasthenia Gravis in Mice. Neuroscience, 373, 113–121. https://doi.org/10.1016/j.neuroscience.2018.01.015
Bergamin E, Hallock PT, Burden SJ, Hubbard SR (2010) The cytoplasmic adaptor protein Dok7 activates the receptor tyrosine kinase MuSK via dimerization. Mol Cell 39:100–109
Challis RC, Ravindra Kumar S, Chan KY, Challis C, Beadle K, Jang MJ, Kim HM, Rajendran PS et al (2019) Systemic AAV vectors for widespread and targeted gene delivery in rodents. Nat Protoc 14(2):379–414. https://doi.org/10.1038/s41596-018-0097-3
Wang Z, Deng X, Zou W, Engelhardt JF, Yan Z, Qiu J (2017) Human bocavirus 1 is a novel helper for adeno-associated virus replication. J Virol 91:e00710–e00717. https://doi.org/10.1128/JVI.00710-17
Katwal AB, Konkalmatt PR, Piras BA, Hazarika S, Li SS, John Lye R, Sanders JM, Ferrante EA et al (2013) Adeno-associated virus serotype 9 efficiently targets ischemic skeletal muscle following systemic delivery. Gene Ther 20(9):930–8. https://doi.org/10.1038/gt.2013.16
Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X (2005) Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol 23(3):321–328. https://doi.org/10.1038/nbt1073
Bauder AR, & Ferguson TA (2012b) Reproducible mouse sciatic nerve crush and subsequent assessment of regeneration by whole mount muscle analysis. J Visualized Exp, 60. https://doi.org/10.3791/3606
Ma CHE, Omura T, Cobos EJ, Latrémolière A, Ghasemlou N, Brenner GJ, van Veen E, Barrett L et al (2011) Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Investig 121(11):4332–4347. https://doi.org/10.1172/jci58675
Liang C, Tao Y, Shen C, Tan Z, Xiong WC, Mei L (2012) Erbin is required for myelination in regenerated axons after injury. J Neurosci 32(43):15169–15180. https://doi.org/10.1523/jneurosci.2466-12.2012
Bridge PM, Ball DJ, Mackinnon SE, Nakao Y, Brandt K, Hunter DA, Hertl C (1994) Nerve crush injuries—a model for axonotmesis. Exp Neurol 127:284–290
Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, Trapp BD, Yan R (2008) Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J 22:2970–2980
Wu H, Lu Y, Shen C, Patel N, Gan L et al (2012) Distinct roles of muscle and motoneuron LRP4 in neuromuscular junction formation. Neuron 75:94–107
Magill CK, Tong A, Kawamura D, Hayashi A, Hunter DA, Parsadanian A, Mackinnon SE, Myckatyn TM (2007) Reinnervation of the tibialis anterior following sciatic nerve crush injury: a confocal microscopic study in transgenic mice. Exp Neurol 207(1):64–74. https://doi.org/10.1016/j.expneurol.2007.05.028
Gao N, Zhao K, Cao Y, Ren X, Jing H, Xing G, Xiong WC, Mei L (2020) A role of lamin A/C in preventing neuromuscular junction decline in mice. J Neurosci. 40(38):7203–7215. https://doi.org/10.1523/JNEUROSCI.0443-20.2020
Zhao K, Shen C, Lu Y, Huang Z, Li L, Rand CD, Pan J, Sun XD, Tan Z, Wang H, Xing G, Cao Y, Hu G, Zhou J, Xiong WC, & Mei L (2017d) Muscle yap is a regulator of neuromuscular junction formation and regeneration. The Journal of Neuroscience, 37(13):3465–3477. https://doi.org/10.1523/jneurosci.2934-16.2017
Yu Z, Zhang M, Jing H, Chen P, Cao R, Pan J, Luo B, Yu Y, Quarles BM, Xiong W, Rivner MH, & Mei L (2021) Characterization of LRP4/Agrin antibodies from a patient with myasthenia gravis. Neurology, 97(10):e975–e987. https://doi.org/10.1212/wnl.0000000000012463
Longo PA, Kavran JM, Kim MS, Leahy DJ (2013) Transient mammalian cell transfection with polyethylenimine (PEI). Methods Enzymol 529:227–240. https://doi.org/10.1016/B978-0-12-418687-3.00018-5
Li L, Cao Y, Wu H, Ye X, Zhu Z, Xing G, Shen C, Barik A et al (2016) Enzymatic activity of the scaffold protein rapsyn for synapse formation. Neuron. 92(5):1007–1019. https://doi.org/10.1016/j.neuron.2016.10.023
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Wu H, Lu Y, Barik A, Joseph A, Taketo MM, Xiong WC, Mei L (2012) β-Catenin gain of function in muscles impairs neuromuscular junction formation. Development 139:2392–2404. https://doi.org/10.1242/dev.080705pmid:22627288
Birks R, Katz B, Miledi R (1960) Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol 150:45168
Burden SJ (1998) The formation of neuromuscular synapses. Genes Dev 12(2):133–148
Valenzuela DM, Stitt TN, DiStefano PS, Rojas E, Mattsson K, Compton DL, Nunez L, Park JS et al (1995) Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron 15:573–584
Hubbard S, Gnanasambandan K (2013) Structure and activation of MuSK, a receptor tyrosine kinase central to neuromuscular junction formation. Biochem Biophys Acta (BBA) Proteins Proteomics 1834(10):2166–2169
Tezuka T, Inoue A, Hoshi T, Weatherbee SD, Burgess RW, Ueta R, Yamanashi Y (2014) The MuSK activator agrin has a separate role essential for postnatal maintenance of neuromuscular synapses. Proc Natl Acad Sci U S A 111:16556–16561
Hakim CH, Wasala NB, Duan D (2013) Evaluation of muscle function of the extensor digitorum longus muscle ex vivo and tibialis anterior muscle in situ in mice. J Visualized Exp : JoVE 72:50183. https://doi.org/10.3791/50183
Kaifer KA, Villalón E, Smith CE, Simon ME, Marquez J, Hopkins AE, Morcos TI, Lorson CL (2020) AAV9-DOK7 gene therapy reduces disease severity in Smn SMA model mice. Biochem Biophys Res Commun 530(1):107–114. https://doi.org/10.1016/j.bbrc.2020.07.031
Ueta R, Sugita S, Minegishi Y, Shimotoyodome A, Ota N, Ogiso N, Eguchi T, Yamanashi Y (2020) DOK7 gene therapy enhances neuromuscular junction innervation and motor function in aged mice. iScience 23(8):101385. https://doi.org/10.1016/j.isci.2020.101385
Miyoshi S, Tezuka T, Arimura S, Tomono T, Okada T, Yamanashi Y (2017) DOK7 gene therapy enhances motor activity and life span in ALS model mice. EMBO Mol Med 9(7):880–889. https://doi.org/10.15252/emmm.201607298
Valdez G, Tapia JC, Kang H, Clemenson GD, Gage FH, Lichtman JW, Sanes JR (2010) Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci 107(33):14863–14868. https://doi.org/10.1073/pnas.1002220107
Taylor JP, Brown RH, Cleveland DW (2016) Decoding ALS: from genes to mechanism. Nature 539(7628):197–206. https://doi.org/10.1038/nature20413
Vinsant S, Mansfield C, Jimenez-Moreno R, Del Gaizo MV, Yoshikawa M, Hampton TG, Prevette D, Caress J et al (2013) Characterization of early pathogenesis in the SOD1(G93A) mouse model of ALS: part II, results and discussion. Brain Behav 3(4):431–57. https://doi.org/10.1002/brb3.142
Perez-Garcia MJ, Burden SJ (2012) Inceasing MuSK activity delays denervation and improves motor function in ALS mice. Cell Rep 2:497–502
Cantor S, Zhang W, Delestrée N, Remédio L, Mentis GZ, & Burden SJ (2018b) Preserving neuromuscular synapses in ALS by stimulating MuSK with a therapeutic agonist antibody. ELife 7. https://doi.org/10.7554/elife.34375
Sengupta-Ghosh A, Dominguez SL, Xie L, Barck KH, Jiang Z, Earr T, Imperio J, Phu L et al (2019) Muscle specific kinase (MuSK) activation preserves neuromuscular junctions in the diaphragm but is not sufficient to provide a functional benefit in the SOD1G93A mouse model of ALS. Neurobiol Dis 124:340–352. https://doi.org/10.1016/j.nbd.2018.12.002
Sumner CJ, Paushkin S, & Ko CP (2016) Spinal Muscular Atrophy: Disease Mechanisms and Therapy. Elsevier Science
Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C et al (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80(1):155–165. https://doi.org/10.1016/0092-8674(95)90460-3 (PMID: 7813012)
Dachs E, Hereu M, Piedrafita L, Casanovas A, Calderó J, Esquerda JE (2011) Defective neuromuscular junction organization and postnatal myogenesis in mice with severe spinal muscular atrophy. J Neuropathol Exp Neurol 70(6):444–461. https://doi.org/10.1097/NEN.0b013e31821cbd8b (PMID: 21572339)
Ling KK, Lin MY, Zingg B, Feng Z, Ko CP (2010) Synaptic defects in the spinal and neuromuscular circuitry in a mouse model of spinal muscular atrophy. PLoS ONE 5(11):e15457. https://doi.org/10.1371/journal.pone.0015457
Kong L, Wang X, Choe DW, Polley M, Burnett BG et al (2009) Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J Neurosci 29:842–851
Murray LM, Comley LH, Thomson D, Parkinson N, Talbot K et al (2008) Selective vulnerability of motor neurons and dissociation of pre- and post-synaptic pathology at the neuromuscular junction in mouse models of spinal muscular atrophy. Hum Mol Genet 17:949–962
Kariya S, Park GH, Maeno-Hikichi Y, Leykekhman O, Lutz C, Arkovitz MS, Landmesser LT, Monani UR (2008) Reduced SMN protein impairs maturation of the neuromuscular junctions in mouse models of spinal muscular atrophy. Hum Mol Genet. 17(16):2552–69. https://doi.org/10.1093/hmg/ddn156
Cifuentes-Diaz C, Nicole S, Velasco ME, Borra-Cebrian C, Panozzo C et al (2002) Neurofilament accumulation at the motor endplate and lack of axonal sprouting in a spinal muscular atrophy mouse model. Hum Mol Genet 11:1439–1447
Kim JK, Caine C, Awano T, Herbst R, Monani UR (2017) Motor neuronal repletion of the NMJ organizer, Agrin, modulates the severity of the spinal muscular atrophy disease phenotype in model mice. Hum Mol Genet 26(13):2377–2385. https://doi.org/10.1093/hmg/ddx124.
Boido M, De Amicis E, Valsecchi V, Trevisan M, Ala U, Ruegg MA, Hettwer S, Vercelli A (2018) Increasing agrin function antagonizes muscle atrophy and motor impairment in spinal muscular atrophy. Front Cell Neurosci 30(12):17. https://doi.org/10.3389/fncel.2018.00017
Arimura S, Okada T, Tezuka T, Chiyo T, Kasahara Y, Yoshimura T, Motomura M, Yoshida N et al (2014) Neuromuscular disease DOK7 gene therapy benefits mouse models of diseases characterized by defects in the neuromuscular junction. Science 345(6203):1505–1508. https://doi.org/10.1126/science.1250744
Burden SJ, Sargent PB, McMahan UJ (1979) Acetylcholine receptors in regenerating muscle accumulate at original synaptic sites in the absence of the nerve. J Cell Biol 82(2):412–425. https://doi.org/10.1083/jcb.82.2.412
Sanes JR, Marshall LM, McMahan UJ (1978) Reinnervation of muscle fiber basal lamina after removal of myofibers Differentiation of regenerating axons at original synaptic sites. J Cell Biol 78(1):176–198. https://doi.org/10.1083/jcb.78.1.176
Hesser BA, Henschel O, Witzemann V (2006) Synapse disassembly and formation of new synapses in postnatal muscle upon conditional inactivation of MuSK. Mol Cell Neurosci 31:470–480
Kong XC, Barzaghi P, Ruegg MA (2004) Inhibition of synapse assembly in mammalian muscle in vivo by RNA interference. EMBO Rep 5:183–188
Eguchi T, Tezuka T, Miyoshi S, Yamanashi Y (2016) Postnatal knockdown of dok-7 gene expression in mice causes structural defects in neuromuscular synapses and myasthenic pathology. Genes Cells 21(6):670–676. https://doi.org/10.1111/gtc.12370 (Epub 2016 Apr 18 PMID: 27091576)
Beeson D, Higuchi O, Palace J, Cossins J, Spearman H, Maxwell S, Newsom-Davis J, Burke G et al (2006) Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science 313(5795):1975–1978. https://doi.org/10.1126/science.1130837
Tinel J (1971) The sign of “Tingling” in lesions of the peripheral nerves. Arch Neurol 24(6):574–575. https://doi.org/10.1001/archneur.1971.00480360108016
Acknowledgements
We appreciate Mei and Xiong laboratory members of Case Western Reserve University for constructive discussions.
Funding
This work was supported by the VA Merit Award (5I01BX001020-08).
Author information
Authors and Affiliations
Contributions
Lin Mei and Wen-Cheng Xiong contributed to the study conception and design. Ethan Kosco performed all experimental work, including viral production and injection, cell transfection, western blots, mouse sciatic nerve injury surgeries, muscle teasing, immunostaining, and confocal imaging under the supervision of Ivy Samuels. Hongyang Jing and Peng Chen contributed to viral production. The first draft of the manuscript was written by Ethan Kosco, and Lin Mei commented and revised it critically before final approval. Lin Mei Lin Mei and Ivy Samuels provided essential comments on figures created by Ethan Kosco. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Experimental procedures and animal care were approved by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University and the Louis Stokes Veterans Affairs Medical Center.
Consent to Participate
Not applicable to this study.
Consent for Publication
Not applicable to this study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kosco, E.D., Jing, H., Chen, P. et al. DOK7 Promotes NMJ Regeneration After Nerve Injury. Mol Neurobiol 60, 1453–1464 (2023). https://doi.org/10.1007/s12035-022-03143-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03143-4