Abstract
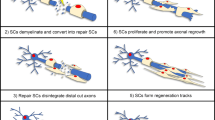
Transcription factors are master regulators of various cellular processes under diverse physiological and pathological conditions. Many transcription factors that are differentially expressed after injury to peripheral nerves play important roles in nerve regeneration. Considering that rapid and timely regrowth of injured axons is a prerequisite for successful target reinnervation, here, we compile transcription factors that mediates axon elongation, including axon growth suppressor Klf4 and axon growth promoters c-Myc, Sox11, STAT3, Atf3, c-Jun, Smad1, C/EBPδ, and p53. Besides neuronal changes, Schwann cell phenotype modulation is also critical for nerve regeneration. The activation of Schwann cells at early time points post injury provides a permissive microenvironment whereas the re-differentiation of Schwann cells at later time points supports myelin sheath formation. Hence, c-Jun and Sox2, two critical drivers for Schwann cell reprogramming, as well as Krox-20 and Sox10, two essential regulators of Schwann cell myelination, are reviewed. These transcription factors may serve as promising targets for promoting the functional recovery of injured peripheral nerves.


Similar content being viewed by others
Data Availability
Not applicable.
References
Yi S, Xu L, Gu X (2019) Scaffolds for peripheral nerve repair and reconstruction. Exp Neurol 319:112761. https://doi.org/10.1016/j.expneurol.2018.05.016
Li R, Liu Z, Pan Y, Chen L, Zhang Z, Lu L (2014) Peripheral nerve injuries treatment: a systematic review. Cell Biochem Biophys 68(3):449–454. https://doi.org/10.1007/s12013-013-9742-1
Garozzo D (2019) Peripheral nerve injuries and their surgical treatment: new perspectives on a changing scenario. Neurol India 67(Supplement):S20–S22. https://doi.org/10.4103/0028-3886.250715
Yi S, Zhang Y, Gu X, Huang L, Zhang K, Qian T, Gu X (2020) Application of stem cells in peripheral nerve regeneration. Burns Trauma 8:tkaa002. https://doi.org/10.1093/burnst/tkaa002
Bhagwat AS, Vakoc CR (2015) Targeting transcription factors in cancer. Trends Cancer 1(1):53–65. https://doi.org/10.1016/j.trecan.2015.07.001
Lambert SA, Jolma A, Campitelli LF, Das PK, Yin Y, Albu M, Chen X, Taipale J et al (2018) The human transcription factors. Cell 172(4):650–665. https://doi.org/10.1016/j.cell.2018.01.029
Lee TI, Young RA (2013) Transcriptional regulation and its misregulation in disease. Cell 152(6):1237–1251. https://doi.org/10.1016/j.cell.2013.02.014
Vaquerizas JM, Kummerfeld SK, Teichmann SA, Luscombe NM (2009) A census of human transcription factors: function, expression and evolution. Nat Rev Genet 10(4):252–263. https://doi.org/10.1038/nrg2538
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. https://doi.org/10.1016/j.cell.2006.07.024
Merrell AJ, Stanger BZ (2016) Adult cell plasticity in vivo: de-differentiation and transdifferentiation are back in style. Nat Rev Mol Cell Biol 17(7):413–425. https://doi.org/10.1038/nrm.2016.24
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872. https://doi.org/10.1016/j.cell.2007.11.019
Varadarajan SG, Hunyara JL, Hamilton NR, Kolodkin AL, Huberman AD (2022) Central nervous system regeneration. Cell 185(1):77–94. https://doi.org/10.1016/j.cell.2021.10.029
Navarro X, Vivo M, Valero-Cabre A (2007) Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 82(4):163–201. https://doi.org/10.1016/j.pneurobio.2007.06.005
Mahar M, Cavalli V (2018) Intrinsic mechanisms of neuronal axon regeneration. Nat Rev Neurosci 19(6):323–337. https://doi.org/10.1038/s41583-018-0001-8
Rishal I, Fainzilber M (2014) Axon-soma communication in neuronal injury. Nat Rev Neurosci 15(1):32–42. https://doi.org/10.1038/nrn3609
Cho Y, Sloutsky R, Naegle KM, Cavalli V (2013) Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell 155(4):894–908. https://doi.org/10.1016/j.cell.2013.10.004
He Z, Jin Y (2016) Intrinsic control of axon regeneration. Neuron 90(3):437–451. https://doi.org/10.1016/j.neuron.2016.04.022
Hao Y, Frey E, Yoon C, Wong H, Nestorovski D, Holzman LB, Giger RJ, DiAntonio A et al (2016) An evolutionarily conserved mechanism for cAMP elicited axonal regeneration involves direct activation of the dual leucine zipper kinase DLK. Elife 5. https://doi.org/10.7554/eLife.14048
Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I et al (2012) Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J 31(6):1350–1363. https://doi.org/10.1038/emboj.2011.494
Xiong X, Wang X, Ewanek R, Bhat P, Diantonio A, Collins CA (2010) Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol 191(1):211–223. https://doi.org/10.1083/jcb.201006039
Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A (2012) Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 74(6):1015–1022. https://doi.org/10.1016/j.neuron.2012.04.028
Luo X, Ribeiro M, Bray ER, Lee DH, Yungher BJ, Mehta ST, Thakor KA, Diaz F et al (2016) Enhanced transcriptional activity and mitochondrial localization of STAT3 co-induce axon regrowth in the adult central nervous system. Cell Rep 15(2):398–410. https://doi.org/10.1016/j.celrep.2016.03.029
Qian T, Wang P, Chen Q, Yi S, Liu Q, Wang H, Wang S, Geng W et al (2018) The dynamic changes of main cell types in the microenvironment of sciatic nerves following sciatic nerve injury and the influence of let-7 on their distribution. RSC Adv 8(72):41181–41191. https://doi.org/10.1039/c8ra08298g
Jessen KR, Mirsky R (2016) The repair Schwann cell and its function in regenerating nerves. J Physiol 594(13):3521–3531. https://doi.org/10.1113/JP270874
Jessen KR, Arthur-Farraj P (2019) Repair Schwann cell update: Adaptive reprogramming, EMT, and stemness in regenerating nerves. Glia 67(3):421–437. https://doi.org/10.1002/glia.23532
Min Q, Parkinson DB, Dun XP (2021) Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia 69(2):235–254. https://doi.org/10.1002/glia.23892
Stierli S, Imperatore V, Lloyd AC (2019) Schwann cell plasticity-roles in tissue homeostasis, regeneration, and disease. Glia 67(11):2203–2215. https://doi.org/10.1002/glia.23643
Su Q, Nasser MI, He J, Deng G, Ouyang Q, Zhuang D, Deng Y, Hu H et al (2022) Engineered Schwann cell-based therapies for injury peripheral nerve reconstruction. Front Cell Neurosci 16:865266. https://doi.org/10.3389/fncel.2022.865266
Chen ZL, Yu WM, Strickland S (2007) Peripheral regeneration. Annu Rev Neurosci 30:209–233. https://doi.org/10.1146/annurev.neuro.30.051606.094337
Chen P, Piao X, Bonaldo P (2015) Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol 130(5):605–618. https://doi.org/10.1007/s00401-015-1482-4
Dun XP, Carr L, Woodley PK, Barry RW, Drake LK, Mindos T, Roberts SL, Lloyd AC et al (2019) Macrophage-derived Slit3 controls cell migration and axon pathfinding in the peripheral nerve bridge. Cell Rep 26(6):1458–1472. https://doi.org/10.1016/j.celrep.2018.12.081
Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M et al (2015) Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 162(5):1127–1139. https://doi.org/10.1016/j.cell.2015.07.021
Liu P, Peng J, Han GH, Ding X, Wei S, Gao G, Huang K, Chang F et al (2019) Role of macrophages in peripheral nerve injury and repair. Neural Regen Res 14(8):1335–1342. https://doi.org/10.4103/1673-5374.253510
He QR, Cong M, Yu FH, Ji YH, Yu S, Shi HY, Ding F (2022) Peripheral nerve fibroblasts secrete neurotrophic factors to promote axon growth of motoneurons. Neural Regen Res 17(8):1833–1840. https://doi.org/10.4103/1673-5374.332159
Wolbert J, Li X, Heming M, Mausberg AK, Akkermann D, Frydrychowicz C, Fledrich R, Groeneweg L et al (2020) Redefining the heterogeneity of peripheral nerve cells in health and autoimmunity. Proc Natl Acad Sci U S A 117(17):9466–9476. https://doi.org/10.1073/pnas.1912139117
Zhang R, Chen S, Wang X, Gu X, Yi S (2021) Cell populations in neonatal rat peripheral nerves identified by single-cell transcriptomics. Glia 69(3):765–778. https://doi.org/10.1002/glia.23928
Saijilafu ZBY, Zhou FQ (2013) Signaling pathways that regulate axon regeneration. Neurosci Bull 29(4):411–420. https://doi.org/10.1007/s12264-013-1357-4
Xu JH, Qin XZ, Zhang HN, Ma YX, Qi SB, Zhang HC, Ma JJ, Fu XY et al (2021) Deletion of Kruppel-like factor-4 promotes axonal regeneration in mammals. Neural Regen Res 16(1):166–171. https://doi.org/10.4103/1673-5374.286978
Schwaiger FW, Hager G, Schmitt AB, Horvat A, Hager G, Streif R, Spitzer C, Gamal S et al (2000) Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT). Eur J Neurosci 12(4):1165–1176. https://doi.org/10.1046/j.1460-9568.2000.00005.x
Bareyre FM, Garzorz N, Lang C, Misgeld T, Buning H, Kerschensteiner M (2011) In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc Natl Acad Sci U S A 108(15):6282–6287. https://doi.org/10.1073/pnas.1015239108
Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T et al (2000) Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: a novel neuronal marker of nerve injury. Mol Cell Neurosci 15(2):170–182. https://doi.org/10.1006/mcne.1999.0814
Gey M, Wanner R, Schilling C, Pedro MT, Sinske D, Knoll B (2016) Atf3 mutant mice show reduced axon regeneration and impaired regeneration-associated gene induction after peripheral nerve injury. Open Biol 6(8). https://doi.org/10.1098/rsob.160091
Jenkins R, Hunt SP (1991) Long-term increase in the levels of c-jun mRNA and jun protein-like immunoreactivity in motor and sensory neurons following axon damage. Neurosci Lett 129(1):107–110. https://doi.org/10.1016/0304-3940(91)90731-8
Lopez de Heredia L, Magoulas C (2013) Lack of the transcription factor C/EBPdelta impairs the intrinsic capacity of peripheral neurons for regeneration. Exp Neurol 239:148–157. https://doi.org/10.1016/j.expneurol.2012.10.012
Tedeschi A, Nguyen T, Puttagunta R, Gaub P, Di Giovanni S (2009) A p53-CBP/p300 transcription module is required for GAP-43 expression, axon outgrowth, and regeneration. Cell Death Differ 16(4):543–554. https://doi.org/10.1038/cdd.2008.175
Shen YY, Zhang RR, Liu QY, Li SY, Yi S (2022) Robust temporal changes of cellular senescence and proliferation after sciatic nerve injury. Neural Regen Res 17(7):1588–1595. https://doi.org/10.4103/1673-5374.330619
Lee J, Shin JE, Lee B, Kim H, Jeon Y, Ahn SH, Chi SW, Cho Y (2020) The stem cell marker Prom1 promotes axon regeneration by down-regulating cholesterol synthesis via Smad signaling. Proc Natl Acad Sci U S A 117(27):15955–15966. https://doi.org/10.1073/pnas.1920829117
Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL (2009) KLF family members regulate intrinsic axon regeneration ability. Science 326(5950):298–301. https://doi.org/10.1126/science.1175737
Chandran V, Coppola G, Nawabi H, Omura T, Versano R, Huebner EA, Zhang A, Costigan M et al (2016) A systems-level analysis of the peripheral nerve intrinsic axonal growth program. Neuron 89(5):956–970. https://doi.org/10.1016/j.neuron.2016.01.034
Belin S, Nawabi H, Wang C, Tang S, Latremoliere A, Warren P, Schorle H, Uncu C et al (2015) Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron 86(4):1000–1014. https://doi.org/10.1016/j.neuron.2015.03.060
Yang SG, Wang XW, Qian C, Zhou FQ (2022) Reprogramming neurons for regeneration: the fountain of youth. Prog Neurobiol 214:102284. https://doi.org/10.1016/j.pneurobio.2022.102284
Ma JJ, Ju X, Xu RJ, Wang WH, Luo ZP, Liu CM, Yang L, Li B et al (2019) Telomerase reverse transcriptase and p53 regulate mammalian peripheral nervous system and CNS axon regeneration downstream of c-Myc. J Neurosci: Off J Soc Neurosci 39(46):9107–9118. https://doi.org/10.1523/JNEUROSCI.0419-19.2019
Shin HY, Kwon MJ, Lee EM, Kim K, Oh YJ, Kim HS, Hwang DH, Kim BG (2021) Role of Myc proto-oncogene as a transcriptional hub to regulate the expression of regeneration-associated genes following preconditioning peripheral nerve injury. J Neurosci: Off J Soc Neurosci 41(3):446–460. https://doi.org/10.1523/JNEUROSCI.1745-20.2020
Julian LM, McDonald AC, Stanford WL (2017) Direct reprogramming with SOX factors: masters of cell fate. Curr Opin Genet Dev 46:24–36. https://doi.org/10.1016/j.gde.2017.06.005
Dodonova SO, Zhu F, Dienemann C, Taipale J, Cramer P (2020) Nucleosome-bound SOX2 and SOX11 structures elucidate pioneer factor function. Nature 580(7805):669–672. https://doi.org/10.1038/s41586-020-2195-y
Masserdotti G, Gillotin S, Sutor B, Drechsel D, Irmler M, Jorgensen HF, Sass S, Theis FJ et al (2015) Transcriptional mechanisms of proneural factors and rest in regulating neuronal reprogramming of astrocytes. Cell Stem Cell 17(1):74–88. https://doi.org/10.1016/j.stem.2015.05.014
Su Z, Zang T, Liu ML, Wang LL, Niu W, Zhang CL (2014) Reprogramming the fate of human glioma cells to impede brain tumor development. Cell Death Dis 5:e1463. https://doi.org/10.1038/cddis.2014.425
Uwanogho D, Rex M, Cartwright EJ, Pearl G, Healy C, Scotting PJ, Sharpe PT (1995) Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech Dev 49(1–2):23–36. https://doi.org/10.1016/0925-4773(94)00299-3
Jankowski MP, Cornuet PK, McIlwrath S, Koerber HR, Albers KM (2006) SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience 143(2):501–514. https://doi.org/10.1016/j.neuroscience.2006.09.010
Jing X, Wang T, Huang S, Glorioso JC, Albers KM (2012) The transcription factor Sox11 promotes nerve regeneration through activation of the regeneration-associated gene Sprr1a. Exp Neurol 233(1):221–232. https://doi.org/10.1016/j.expneurol.2011.10.005
Jankowski MP, McIlwrath SL, Jing X, Cornuet PK, Salerno KM, Koerber HR, Albers KM (2009) Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res 1256:43–54. https://doi.org/10.1016/j.brainres.2008.12.032
Jankowski MP, Miller L, Koerber HR (2018) Increased expression of transcription factor SRY-box-containing gene 11 (Sox11) enhances neurite growth by regulating neurotrophic factor responsiveness. Neuroscience 382:93–104. https://doi.org/10.1016/j.neuroscience.2018.04.037
Tang Y, Luo Y, Jiang Z, Ma Y, Lin CJ, Kim C, Carter MG, Amano T et al (2012) Jak/Stat3 signaling promotes somatic cell reprogramming by epigenetic regulation. Stem cells 30(12):2645–2656. https://doi.org/10.1002/stem.1225
Qiu J, Cafferty WB, McMahon SB, Thompson SW (2005) Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci: Off J Soc Neurosci 25(7):1645–1653. https://doi.org/10.1523/JNEUROSCI.3269-04.2005
Ma JJ, Xu RJ, Qi SB, Wang F, Ma YX, Zhang HC, Xu JH, Qin XZ et al (2019) Regulation of adult mammalian intrinsic axonal regeneration by NF-kappaB/STAT3 signaling cascade. J Cell Physiol 234(12):22517–22528. https://doi.org/10.1002/jcp.28815
Bloechlinger S, Karchewski LA, Woolf CJ (2004) Dynamic changes in glypican-1 expression in dorsal root ganglion neurons after peripheral and central axonal injury. Eur J Neurosci 19(5):1119–1132. https://doi.org/10.1111/j.1460-9568.2004.03262.x
Seijffers R, Allchorne AJ, Woolf CJ (2006) The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci 32(1–2):143–154. https://doi.org/10.1016/j.mcn.2006.03.005
Seijffers R, Mills CD, Woolf CJ (2007) ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci: Off J Soc Neurosci 27(30):7911–7920. https://doi.org/10.1523/JNEUROSCI.5313-06.2007
Renthal W, Tochitsky I, Yang L, Cheng YC, Li E, Kawaguchi R, Geschwind DH, Woolf CJ (2020) Transcriptional reprogramming of distinct peripheral sensory neuron subtypes after axonal injury. Neuron 108(1):128-144.e129. https://doi.org/10.1016/j.neuron.2020.07.026
Ruff CA, Staak N, Patodia S, Kaswich M, Rocha-Ferreira E, Da Costa C, Brecht S, Makwana M et al (2012) Neuronal c-Jun is required for successful axonal regeneration, but the effects of phosphorylation of its N-terminus are moderate. J Neurochem 121(4):607–618. https://doi.org/10.1111/j.1471-4159.2012.07706.x
Tedeschi A (2011) Tuning the orchestra: transcriptional pathways controlling axon regeneration. Front Mol Neurosci 4:60. https://doi.org/10.3389/fnmol.2011.00060
Kiryu-Seo S, Kato R, Ogawa T, Nakagomi S, Nagata K, Kiyama H (2008) Neuronal injury-inducible gene is synergistically regulated by ATF3, c-Jun, and STAT3 through the interaction with Sp1 in damaged neurons. J Biol Chem 283(11):6988–6996. https://doi.org/10.1074/jbc.M707514200
Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK et al (2010) PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci 13(9):1075–1081. https://doi.org/10.1038/nn.2603
Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L et al (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322(5903):963–966. https://doi.org/10.1126/science.1161566
Saijilafu HEM, Liu CM, Jiao Z, Xu WL, Zhou FQ (2013) PI3K-GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nat Commun 4:2690. https://doi.org/10.1038/ncomms3690
Gobrecht P, Leibinger M, Andreadaki A, Fischer D (2014) Sustained GSK3 activity markedly facilitates nerve regeneration. Nat Commun 5:4561. https://doi.org/10.1038/ncomms5561
Zou H, Ho C, Wong K, Tessier-Lavigne M (2009) Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci: Off J Soc Neurosci 29(22):7116–7123. https://doi.org/10.1523/JNEUROSCI.5397-08.2009
Finelli MJ, Wong JK, Zou H (2013) Epigenetic regulation of sensory axon regeneration after spinal cord injury. J Neurosci: Off J Soc Neurosci 33(50):19664–19676. https://doi.org/10.1523/JNEUROSCI.0589-13.2013
Jang EH, Bae YH, Yang EM, Gim Y, Suh HJ, Kim S, Park SM, Park JB et al (2021) Comparing axon regeneration in male and female mice after peripheral nerve injury. J Neurosci Res 99(11):2874–2887. https://doi.org/10.1002/jnr.24955
Patodia S, Raivich G (2012) Role of transcription factors in peripheral nerve regeneration. Front Mol Neurosci 5:8. https://doi.org/10.3389/fnmol.2012.00008
Nadeau S, Hein P, Fernandes KJ, Peterson AC, Miller FD (2005) A transcriptional role for C/EBP beta in the neuronal response to axonal injury. Mol Cell Neurosci 29(4):525–535. https://doi.org/10.1016/j.mcn.2005.04.004
Di Giovanni S, Knights CD, Rao M, Yakovlev A, Beers J, Catania J, Avantaggiati ML, Faden AI (2006) The tumor suppressor protein p53 is required for neurite outgrowth and axon regeneration. EMBO J 25(17):4084–4096. https://doi.org/10.1038/sj.emboj.7601292
Blackmore MG (2012) Molecular control of axon growth: insights from comparative gene profiling and high-throughput screening. Int Rev Neurobiol 105:39–70. https://doi.org/10.1016/B978-0-12-398309-1.00004-4
Wang Q, Gong L, Mao S, Yao C, Liu M, Wang Y, Yang J, Yu B et al (2021) Klf2-Vav1-Rac1 axis promotes axon regeneration after peripheral nerve injury. Exp Neurol 343:113788. https://doi.org/10.1016/j.expneurol.2021.113788
Yi S, Zhang H, Gong L, Wu J, Zha G, Zhou S, Gu X, Yu B (2015) Deep sequencing and bioinformatic analysis of lesioned sciatic nerves after crush injury. PLoS ONE 10(12):e0143491. https://doi.org/10.1371/journal.pone.0143491
Gong L, Wu J, Zhou S, Wang Y, Qin J, Yu B, Gu X, Yao C (2016) Global analysis of transcriptome in dorsal root ganglia following peripheral nerve injury in rats. Biochem Biophys Res Commun 478(1):206–212. https://doi.org/10.1016/j.bbrc.2016.07.067
Arthur-Farraj PJ, Morgan CC, Adamowicz M, Gomez-Sanchez JA, Fazal SV, Beucher A, Razzaghi B, Mirsky R et al (2017) Changes in the coding and non-coding transcriptome and DNA Methylome that define the Schwann cell repair phenotype after nerve injury. Cell Rep 20(11):2719–2734. https://doi.org/10.1016/j.celrep.2017.08.064
Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B et al (2012) c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron 75(4):633–647. https://doi.org/10.1016/j.neuron.2012.06.021
Carr MJ, Johnston AP (2017) Schwann cells as drivers of tissue repair and regeneration. Curr Opin Neurobiol 47:52–57. https://doi.org/10.1016/j.conb.2017.09.003
Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P (1994) Krox-20 controls myelination in the peripheral nervous system. Nature 371(6500):796–799. https://doi.org/10.1038/371796a0
Hung HA, Sun G, Keles S, Svaren J (2015) Dynamic regulation of Schwann cell enhancers after peripheral nerve injury. J Biol Chem 290(11):6937–6950. https://doi.org/10.1074/jbc.M114.622878
Bremer M, Frob F, Kichko T, Reeh P, Tamm ER, Suter U, Wegner M (2011) Sox10 is required for Schwann-cell homeostasis and myelin maintenance in the adult peripheral nerve. Glia 59(7):1022–1032. https://doi.org/10.1002/glia.21173
Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, Feltri ML, Wrabetz L et al (2008) c-Jun is a negative regulator of myelination. J Cell Biol 181(4):625–637. https://doi.org/10.1083/jcb.200803013
Fontana X, Hristova M, Da Costa C, Patodia S, Thei L, Makwana M, Spencer-Dene B, Latouche M et al (2012) c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. J Cell Biol 198(1):127–141. https://doi.org/10.1083/jcb.201205025
Painter MW, Brosius Lutz A, Cheng YC, Latremoliere A, Duong K, Miller CM, Posada S, Cobos EJ et al (2014) Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 83(2):331–343. https://doi.org/10.1016/j.neuron.2014.06.016
Wagstaff LJ, Gomez-Sanchez JA, Fazal SV, Otto GW, Kilpatrick AM, Michael K, Wong LYN, Ma KH (2021) Failures of nerve regeneration caused by aging or chronic denervation are rescued by restoring Schwann cell c-Jun. Elife 10. https://doi.org/10.7554/eLife.62232
Jessen KR, Mirsky R (2021) The role of c-Jun and autocrine signaling loops in the control of repair Schwann cells and regeneration. Front Cell Neurosci 15:820216. https://doi.org/10.3389/fncel.2021.820216
Fazal SV, Gomez-Sanchez JA, Wagstaff LJ, Musner N, Otto G, Janz M, Mirsky R, Jessen KR (2017) Graded elevation of c-Jun in Schwann cells in vivo: gene dosage determines effects on development, remyelination, tumorigenesis, and hypomyelination. J Neurosci: Off J Soc Neurosci 37(50):12297–12313. https://doi.org/10.1523/JNEUROSCI.0986-17.2017
Figlia G (2018) c-Jun in Schwann cells: stay away from extremes. J Neurosci: Off J Soc Neurosci 38(14):3388–3390. https://doi.org/10.1523/JNEUROSCI.0028-18.2018
Jessen KR, Mirsky R (2008) Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia 56(14):1552–1565. https://doi.org/10.1002/glia.20761
Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J (2005) Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies Sox2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci U S A 102(7):2596–2601. https://doi.org/10.1073/pnas.0407836102
Roberts SL, Dun XP, Doddrell RDS, Mindos T, Drake LK, Onaitis MW, Florio F, Quattrini A et al (2017) Sox2 expression in Schwann cells inhibits myelination in vivo and induces influx of macrophages to the nerve. Development 144(17):3114–3125. https://doi.org/10.1242/dev.150656
Parrinello S, Napoli I, Ribeiro S, Wingfield Digby P, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M et al (2010) EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 143(1):145–155. https://doi.org/10.1016/j.cell.2010.08.039
Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D’Antonio M, Mirsky R, Jessen KR (2004) Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol 164(3):385–394. https://doi.org/10.1083/jcb.200307132
Decker L, Desmarquet-Trin-Dinh C, Taillebourg E, Ghislain J, Vallat JM, Charnay P (2006) Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. J Neurosci: Off J Soc Neurosci 26(38):9771–9779. https://doi.org/10.1523/JNEUROSCI.0716-06.2006
Jang SW, LeBlanc SE, Roopra A, Wrabetz L, Svaren J (2006) In vivo detection of Egr2 binding to target genes during peripheral nerve myelination. J Neurochem 98(5):1678–1687. https://doi.org/10.1111/j.1471-4159.2006.04069.x
Jang SW, Srinivasan R, Jones EA, Sun G, Keles S, Krueger C, Chang LW, Nagarajan R et al (2010) Locus-wide identification of Egr2/Krox20 regulatory targets in myelin genes. J Neurochem 115(6):1409–1420. https://doi.org/10.1111/j.1471-4159.2010.07045.x
Tammia M, Mi R, Sluch VM, Zhu A, Chung T, Shinn D, Zack DJ, Hoke A et al (2018) Egr2 overexpression in Schwann cells increases myelination frequency in vitro. Heliyon 4(11):e00982. https://doi.org/10.1016/j.heliyon.2018.e00982
Sock E, Wegner M (2019) Transcriptional control of myelination and remyelination. Glia 67(11):2153–2165. https://doi.org/10.1002/glia.23636
Jones EA, Lopez-Anido C, Srinivasan R, Krueger C, Chang LW, Nagarajan R, Svaren J (2011) Regulation of the PMP22 gene through an intronic enhancer. J Neurosci 31(11):4242–4250. https://doi.org/10.1523/JNEUROSCI.5893-10.2011
Jones EA, Brewer MH, Srinivasan R, Krueger C, Sun G, Charney KN, Keles S, Antonellis A et al (2012) Distal enhancers upstream of the Charcot-Marie-Tooth type 1A disease gene PMP22. Hum Mol Genet 21(7):1581–1591. https://doi.org/10.1093/hmg/ddr595
Kawasaki T, Oka N, Tachibana H, Akiguchi I, Shibasaki H (2003) Oct6, a transcription factor controlling myelination, is a marker for active nerve regeneration in peripheral neuropathies. Acta Neuropathol 105(3):203–208. https://doi.org/10.1007/s00401-002-0630-9
Ghislain J, Charnay P (2006) Control of myelination in Schwann cells: a Krox20 cis-regulatory element integrates Oct6, Brn2 and Sox10 activities. EMBO Rep 7(1):52–58. https://doi.org/10.1038/sj.embor.7400573
Quintes S, Brinkmann BG, Ebert M, Frob F, Kungl T, Arlt FA, Tarabykin V, Huylebroeck D et al (2016) Zeb2 is essential for Schwann cell differentiation, myelination and nerve repair. Nat Neurosci 19(8):1050–1059. https://doi.org/10.1038/nn.4321
Benito C, Davis CM, Gomez-Sanchez JA, Turmaine M, Meijer D, Poli V, Mirsky R, Jessen KR (2017) STAT3 controls the long-term survival and phenotype of repair Schwann cells during nerve regeneration. J Neurosci: Off J Soc Neurosci 37(16):4255–4269. https://doi.org/10.1523/JNEUROSCI.3481-16.2017
Yi S, Tang X, Yu J, Liu J, Ding F, Gu X (2017) Microarray and qPCR analyses of Wallerian degeneration in rat sciatic nerves. Front Cell Neurosci 11:22. https://doi.org/10.3389/fncel.2017.00022
Zhang F, Gu X, Yi S, Xu H (2019) Dysregulated transcription factor TFAP2A after peripheral nerve injury modulated Schwann cell phenotype. Neurochem Res 44(12):2776–2785. https://doi.org/10.1007/s11064-019-02898-y
Zhang L, Johnson D, Johnson JA (2013) Deletion of Nrf2 impairs functional recovery, reduces clearance of myelin debris and decreases axonal remyelination after peripheral nerve injury. Neurobiol Dis 54:329–338. https://doi.org/10.1016/j.nbd.2013.01.003
Tao J, Miao R, Liu G, Qiu X, Yang B, Tan X, Liu L, Long J et al (2022) Spatiotemporal correlation between HIF-1alpha and bone regeneration. FASEB J 36(10):e22520. https://doi.org/10.1096/fj.202200329RR
Haastert K, Grothe C (2007) Gene therapy in peripheral nerve reconstruction approaches. Curr Gene Ther 7(3):221–228. https://doi.org/10.2174/156652307780859035
Bushweller JH (2019) Targeting transcription factors in cancer - from undruggable to reality. Nat Rev Cancer 19(11):611–624. https://doi.org/10.1038/s41568-019-0196-7
Dey A, Varelas X, Guan KL (2020) Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat Rev Drug Discov 19(7):480–494. https://doi.org/10.1038/s41573-020-0070-z
Funding
This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
Yunsong Zhang prepared the figures. Yunsong Zhang, Qian Zhao, and Qianqian Chen collected the data. Lingchi Xu and Sheng Yi designed the study and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Zhao, Q., Chen, Q. et al. Transcriptional Control of Peripheral Nerve Regeneration. Mol Neurobiol 60, 329–341 (2023). https://doi.org/10.1007/s12035-022-03090-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-03090-0