Abstract
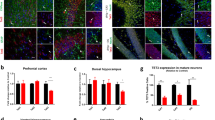
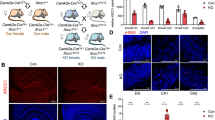
TET enzymes (TET1-3) are dioxygenases that oxidize 5-methylcytosine (5mC) into 5-hydroxymethylcytosine (5hmC) and are involved in the DNA demethylation process. In line with the observed 5hmC abundance in the brain, Tet genes are highly transcribed, with Tet3 being the predominant member. We have previously shown that Tet3 conditional deletion in the brain of male mice was associated with anxiety-like behavior and impairment in hippocampal-dependent spatial orientation. In the current study, we addressed the role of Tet3 in female mice and its impact on behavior, using in vivo conditional and inducible deletion from post-mitotic neurons. Our results indicate that conditional and inducible deletion of Tet3 in female mice increases anxiety-like behavior and impairs both spatial orientation and short-term memory. At the molecular level, we identified upregulation of immediate-early genes, particularly Npas4, in both the dorsal and ventral hippocampus and in the prefrontal cortex. This study shows that deletion of Tet3 in female mice differentially affects behavioral dimensions as opposed to Tet3 deletion in males, highlighting the importance of studying both sexes in behavioral studies. Moreover, it contributes to expand the knowledge on the role of epigenetic regulators in brain function and behavioral outcome.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Feng J, Fouse S, Fan G (2007) Epigenetic regulation of neural gene expression and neuronal function. Pediatr Res 61(5 Pt 2):58r–63r. https://doi.org/10.1203/pdr.0b013e3180457635
Snijders C, Bassil KC, de Nijs L (2018) Methodologies of neuroepigenetic research: background, challenges and future perspectives. Prog Mol Biol Transl Sci 158:15–27. https://doi.org/10.1016/bs.pmbts.2018.04.009
Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y (2010) Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466(7310):1129–1133. https://doi.org/10.1038/nature09303
Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y (2011) Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 333(6047):1300–1303. https://doi.org/10.1126/science.1210597
Liu S, Wang J, Su Y, Guerrero C, Zeng Y, Mitra D, Brooks PJ, Fisher DE, Song H, Wang Y (2013) Quantitative assessment of Tet-induced oxidation products of 5-methylcytosine in cellular and tissue DNA. Nucleic Acids Res 41(13):6421–6429. https://doi.org/10.1093/nar/gkt360
Branco MR, Ficz G, Reik W (2011) Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet 13(1):7–13. https://doi.org/10.1038/nrg3080
Hahn MA, Szabó PE, Pfeifer GP (2014) 5-Hydroxymethylcytosine: a stable or transient DNA modification? Genomics 104(5):314–323. https://doi.org/10.1016/j.ygeno.2014.08.015
Guo JU, Su Y, Zhong C, Ming GL, Song H (2011) Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145(3):423–434. https://doi.org/10.1016/j.cell.2011.03.022
Zhang RR, Cui QY, Murai K, Lim YC, Smith ZD, Jin S, Ye P, Rosa L, Lee YK, Wu HP, Liu W, Xu ZM, Yang L, Ding YQ, Tang F, Meissner A, Ding C, Shi Y, Xu GL (2013) Tet1 regulates adult hippocampal neurogenesis and cognition. Cell Stem Cell 13(2):237–245. https://doi.org/10.1016/j.stem.2013.05.006
Gontier G, Iyer M, Shea JM, Bieri G, Wheatley EG, Ramalho-Santos M, Villeda SA (2018) Tet2 rescues age-related regenerative decline and enhances cognitive function in the adult mouse brain. Cell Rep 22(8):1974–1981. https://doi.org/10.1016/j.celrep.2018.02.001
Rudenko A, Dawlaty MM, Seo J, Cheng AW, Meng J, Le T, Faull KF, Jaenisch R, Tsai LH (2013) Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron 79(6):1109–1122. https://doi.org/10.1016/j.neuron.2013.08.003
Kumar D, Aggarwal M, Kaas GA, Lewis J, Wang J, Ross DL, Zhong C, Kennedy A, Song H, Sweatt JD (2015) Tet1 oxidase regulates neuronal gene transcription, active DNA Hydroxy-methylation, object location memory, and threat recognition memory. Neuroepigenetics 4:12–27. https://doi.org/10.1016/j.nepig.2015.10.002
Kaas GA, Zhong C, Eason DE, Ross DL, Vachhani RV, Ming GL, King JR, Song H, Sweatt JD (2013) TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation. Neuron 79(6):1086–1093. https://doi.org/10.1016/j.neuron.2013.08.032
Li X, Wei W, Zhao QY, Widagdo J, Baker-Andresen D, Flavell CR, D’Alessio A, Zhang Y, Bredy TW (2014) Neocortical Tet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation. Proc Natl Acad Sci U S A 111(19):7120–7125. https://doi.org/10.1073/pnas.1318906111
Antunes C, Sousa N, Pinto L, Marques CJ (2019) TET enzymes in neurophysiology and brain function. Neurosci Biobehav Rev 102:337–344. https://doi.org/10.1016/j.neubiorev.2019.05.006
Szwagierczak A, Bultmann S, Schmidt CS, Spada F, Leonhardt H (2010) Sensitive enzymatic quantification of 5-hydroxymethylcytosine in genomic DNA. Nucleic Acids Res 38(19):e181. https://doi.org/10.1093/nar/gkq684
MacArthur IC, Dawlaty MM (2021) TET Enzymes and 5-hydroxymethylcytosine in neural progenitor cell biology and neurodevelopment. Front Cell Dev Biol 9:645335. https://doi.org/10.3389/fcell.2021.645335
Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG, Iqbal K, Shi YG, Deng Z, Szabo PE, Pfeifer GP, Li J, Xu GL (2011) The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 477(7366):606–610. https://doi.org/10.1038/nature10443
Kremer EA, Gaur N, Lee MA, Engmann O, Bohacek J, Mansuy IM (2018) Interplay between TETs and microRNAs in the adult brain for memory formation. Sci Rep 8(1):1678. https://doi.org/10.1038/s41598-018-19806-z
Yu H, Su Y, Shin J, Zhong C, Guo JU, Weng YL, Gao F, Geschwind DH, Coppola G, Ming GL, Song H (2015) Tet3 regulates synaptic transmission and homeostatic plasticity via DNA oxidation and repair. Nat Neurosci 18(6):836–843. https://doi.org/10.1038/nn.4008
Antunes C, Da Silva JD, Guerra-Gomes S, Alves ND, Ferreira F, Loureiro-Campos E, Branco MR, Sousa N, Reik W, Pinto L, Marques CJ (2021) Tet3 ablation in adult brain neurons increases anxiety-like behavior and regulates cognitive function in mice. Mol Psychiatry 26(5):1445–1457. https://doi.org/10.1038/s41380-020-0695-7
Ratnu VS, Emami MR, Bredy TW (2017) Genetic and epigenetic factors underlying sex differences in the regulation of gene expression in the brain. J Neurosci Res 95(1–2):301–310. https://doi.org/10.1002/jnr.23886
Peat JR, Dean W, Clark SJ, Krueger F, Smallwood SA, Ficz G, Kim JK, Marioni JC, Hore TA, Reik W (2014) Genome-wide bisulfite sequencing in zygotes identifies demethylation targets and maps the contribution of TET3 oxidation. Cell Rep 9(6):1990–2000. https://doi.org/10.1016/j.celrep.2014.11.034
Santos F, Peat J, Burgess H, Rada C, Reik W, Dean W (2013) Active demethylation in mouse zygotes involves cytosine deamination and base excision repair. Epigenetics Chromatin 6(1):39. https://doi.org/10.1186/1756-8935-6-39
Byers SL, Wiles MV, Dunn SL, Taft RA (2012) Mouse estrous cycle identification tool and images. PLoS ONE 7(4):e35538. https://doi.org/10.1371/journal.pone.0035538
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Harris KM, Jensen FE, Tsao B (1992) Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci 12(7):2685–2705
Liu XB, Murray KD (2012) Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: location, location, location. Epilepsia 53(Suppl 1):45–52. https://doi.org/10.1111/j.1528-1167.2012.03474.x
Graziano A, Petrosini L, Bartoletti A (2003) Automatic recognition of explorative strategies in the Morris water maze. J Neurosci Methods 130(1):33–44
Feng J, Pena CJ, Purushothaman I, Engmann O, Walker D, Brown AN, Issler O, Doyle M, Harrigan E, Mouzon E, Vialou V, Shen L, Dawlaty MM, Jaenisch R, Nestler EJ (2017) Tet1 in nucleus accumbens opposes depression- and anxiety-like behaviors. Neuropsychopharmacology 42(8):1657–1669. https://doi.org/10.1038/npp.2017.6
Beck DB, Petracovici A, He C, Moore HW, Louie RJ, Ansar M, Douzgou S, Sithambaram S, Cottrell T, Santos-Cortez RLP, Prijoles EJ, Bend R, Keren B, Mignot C, Nougues MC, Ounap K, Reimand T, Pajusalu S, Zahid M, Saqib MAN, Buratti J, Seaby EG, McWalter K, Telegrafi A, Baldridge D, Shinawi M, Leal SM, Schaefer GB, Stevenson RE, Banka S, Bonasio R, Fahrner JA (2020) Delineation of a human mendelian disorder of the DNA demethylation machinery: TET3 deficiency. Am J Hum Genet 106(2):234–245. https://doi.org/10.1016/j.ajhg.2019.12.007
Kandel ER, Dudai Y, Mayford MR (2014) The molecular and systems biology of memory. Cell 157(1):163–186. https://doi.org/10.1016/j.cell.2014.03.001
Preston AR, Eichenbaum H (2013) Interplay of hippocampus and prefrontal cortex in memory. Current Biol CB 23(17):R764–R773. https://doi.org/10.1016/j.cub.2013.05.041
Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM (2015) Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci 18(5):690–697. https://doi.org/10.1038/nn.3988
Loebrich S, Nedivi E (2009) The function of activity-regulated genes in the nervous system. Physiol Rev 89(4):1079–1103. https://doi.org/10.1152/physrev.00013.2009
Coutellier L, Beraki S, Ardestani PM, Saw NL, Shamloo M (2012) Npas4: a neuronal transcription factor with a key role in social and cognitive functions relevant to developmental disorders. PLoS ONE 7(9):e46604. https://doi.org/10.1371/journal.pone.0046604
Tovote P, Fadok JP, Luthi A (2015) Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16(6):317–331. https://doi.org/10.1038/nrn3945
Wang L, Li MY, Qu C, Miao WY, Yin Q, Liao J, Cao HT, Huang M, Wang K, Zuo E, Peng G, Zhang SX, Chen G, Li Q, Tang K, Yu Q, Li Z, Wong CC, Xu G, Jing N, Yu X, Li J (2017) CRISPR-Cas9-mediated genome editing in one blastomere of two-cell embryos reveals a novel Tet3 function in regulating neocortical development. Cell Res 27(6):815–829. https://doi.org/10.1038/cr.2017.58
Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, Zhou P, Ong SK, Wright MA, Zweifel L, Paninski L, Hen R, Kheirbek MA (2018) Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97(3):670-683.e676. https://doi.org/10.1016/j.neuron.2018.01.016
Yoshida K, Drew MR, Mimura M, Tanaka KF (2019) Serotonin-mediated inhibition of ventral hippocampus is required for sustained goal-directed behavior. Nat Neurosci 22(5):770–777. https://doi.org/10.1038/s41593-019-0376-5
Andersson KB, Winer LH, Mork HK, Molkentin JD, Jaisser F (2010) Tamoxifen administration routes and dosage for inducible Cre-mediated gene disruption in mouse hearts. Transgenic Res 19(4):715–725. https://doi.org/10.1007/s11248-009-9342-4
Acknowledgements
The authors would like to thank Wolf Reik and Julian Peat (Babraham Institute, Cambridge, UK) for providing the Tet3 floxed mice and Nuno Sousa (School of Medicine, University of Minho, Portugal) for critical reading of the manuscript.
Funding
This work was supported by National Funds through Foundation for Science and Technology (FCT) fellowships (PD/BD/106049/2015 to CA, PD/BD/128074/2016 to JDS, SFRH/BD/131278/2017 to ELC, SFRH/BD/101298/2014 to SGM, IF/01079/2014 to LP, IF/00047/2012 and CEECIND/00371/2017 to CJM); FCT project grant (PTDC/BIA-BCM/121276/2010) to C.J.M; EpiGeneSys Small Collaborative project to LP; BIAL Foundation Grant 427/14 to LP; and Nature Research Award for Driving Global Impact – 2019 Brain Sciences to LP. It was also co-funded by the Life and Health Sciences Research Institute (ICVS) and by the FEDER (NORTE-01–0145-FEDER-000013), through the Competitiveness Internationalization Operational Program (POCI), and by National funds, through the Foundation for Science and Technology (FCT) – project UIDB/50026/2020 and UIDP/50026/2020. Moreover, this work has been funded by ICVS Scientific Microscopy Platform, member of the national infrastructure PPBI – Portuguese Platform of Bioimaging (PPBI-POCI-01–0145-FEDER-022122; by National funds, through the Foundation or Science and Technology (FCT) – project UIDB/50026/2020 and UIDP/50026/2020).
Author information
Authors and Affiliations
Contributions
C.A designed the study, performed the experiments, analyzed the data, and wrote the manuscript; J.D.S. performed the statistical analysis; S.G.G. and N.D.A. helped with the behavioral tests and respective analysis; E.L.C. helped with the behavioral tests; L.P. and C.J.M. designed the study and wrote the final manuscript. All authors revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
All experiments were conducted in accordance with EU Directive 2010/63/EU and NIH guidelines on animal care and experimentation and were approved by the Portuguese Authorities (DGAV) with the project reference 0421/000/000/2017.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Antunes, C., Da Silva, J.D., Guerra-Gomes, S. et al. Tet3 Deletion in Adult Brain Neurons of Female Mice Results in Anxiety-like Behavior and Cognitive Impairments. Mol Neurobiol 59, 4892–4901 (2022). https://doi.org/10.1007/s12035-022-02883-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02883-7