Abstract
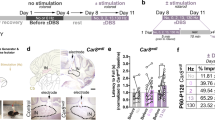
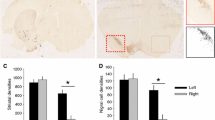
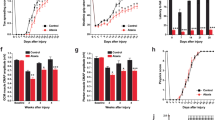
Cerebellum is one of the major targets of autoimmunity and cerebellar damage that leads to ataxia characterized by the loss of fine motor coordination and balance, with no treatment available. Deep brain stimulation (DBS) could be a promising treatment for ataxia but has not been extensively investigated. Here, our study aims to investigate the use of interposed nucleus of deep cerebellar nuclei (IN-DCN) for ataxia. We first characterized ataxia-related motor symptom of a Purkinje cell (PC)-specific LIM homeobox (Lhx)1 and Lhx5 conditional double knockout mice by motor coordination tests, and spontaneous electromyogram (EMG) recording. To validate IN-DCN as a target for DBS, in vivo local field potential (LFP) multielectrode array recording of IN-DCN revealed abnormal LFP amplitude surges in PCs. By synchronizing the EMG and IN-DCN recordings (neurospike and LFP) with high-speed video recordings, ataxia mice showed poorly coordinated movements associated with low EMG amplitude and aberrant IN-DCN neural firing. To optimize IN-DCN-DBS for ataxia, we tested DBS parameters from low (30 Hz) to high stimulation frequency (130 or 150 Hz), and systematically varied pulse width values (60 or 80 µs) to maximize motor symptom control in ataxia mice. The optimal IN-DCN-DBS parameter reversed motor deficits in ataxia mice as detected by animal behavioral tests and EMG recording. Mechanistically, cytokine array analysis revealed that anti-inflammatory cytokines such as interleukin (IL)-13 and IL-4 were upregulated after IN-DCN-DBS, which play key roles in neural excitability. As such, we show that IN-DCN-DBS is a promising treatment for ataxia and possibly other movement disorders alike.






Similar content being viewed by others
Data Availability
The datasets and supporting materials generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AD:
-
Alzheimer’s disease
- AP:
-
Anteroposterior
- BOSS:
-
Blackrock offline spike sorting
- CSF:
-
Cerebrospinal fluid
- DBS :
-
Deep brain stimulation
- DCN:
-
Deep cerebellar nuclei
- DKO:
-
Double knockout
- DN:
-
Dentate nucleus
- DV:
-
Dorsoventral
- EMG:
-
Electromyogram
- GAD:
-
Glutamic acid decarboxylase
- GCM:
-
Gastrocnemius muscle
- GPi :
-
Globus pallidus pars interna
- G-CSF:
-
Granulocyte-colony stimulating factor
- IL:
-
Interleukin
- IN:
-
Interposed nucleus
- IN-DCN:
-
Interposed nucleus of deep cerebellar nuclei
- IN-DCN-DBS:
-
Interposed nucleus-deep cerebellar nuclei- deep brain stimulation
- IR:
-
Infrared
- LFP:
-
Local field potential
- Lhx :
-
LIM homeobox
- MATLAB:
-
Matrix laboratory
- MEA:
-
Multielectrode array
- ML:
-
Mediolateral
- MS:
-
Multiple sclerosis
- PC:
-
Purkinje cell
- PD:
-
Parkinson’s disease
- PCA:
-
Principal component analysis
- RMS :
-
Root mean square
- SCF:
-
Stem cell factor
- STN:
-
Subthalamic nucleus
- TBI:
-
Traumatic brain injury
References
Bensmaia SJ, Miller LE (2014) Restoring sensorimotor function through intracortical interfaces: progress and looming challenges. Nat Rev Neurosci 15(5):313–325. https://doi.org/10.1038/nrn3724
Li Q, Ke Y, Chan DC, Qian ZM, Yung KK, Ko H, Arbuthnott GW, Yung WH (2012) Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron 76(5):1030–1041. https://doi.org/10.1016/j.neuron.2012.09.032
Xu W, Russo GS, Hashimoto T, Zhang J, Vitek JL (2008) Subthalamic nucleus stimulation modulates thalamic neuronal activity. J Neurosci 28(46):11916–11924. https://doi.org/10.1523/JNEUROSCI.2027-08.2008
Cagnan H, Denison T, McIntyre C, Brown P (2019) Emerging technologies for improved deep brain stimulation. Nat Biotechnol 37(9):1024–1033. https://doi.org/10.1038/s41587-019-0244-6
Lozano AM, Lipsman N (2013) Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 77(3):406–424. https://doi.org/10.1016/j.neuron.2013.01.020
Fasano A, Lozano AM (2015) Deep brain stimulation for movement disorders: 2015 and beyond. Curr Opin Neurol 28(4):423–436. https://doi.org/10.1097/WCO.0000000000000226
Sarna JR, Hawkes R (2003) Patterned Purkinje cell death in the cerebellum. Prog Neurobiol 70(6):473–507. https://doi.org/10.1016/s0301-0082(03)00114-x
Taroni F, DiDonato S (2004) Pathways to motor incoordination: the inherited ataxias. Nat Rev Neurosci 5(8):641–655. https://doi.org/10.1038/nrn1474
Klockgether T, Mariotti C, Paulson HL (2019) Spinocerebellar ataxia Nat Rev Dis Primers 5(1):24. https://doi.org/10.1038/s41572-019-0074-3
Gao Z, van Beugen BJ, De Zeeuw CI (2012) Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci 13(9):619–635. https://doi.org/10.1038/nrn3312
Hoogland TM, De Gruijl JR, Witter L, Canto CB, De Zeeuw CI (2015) Role of synchronous activation of cerebellar purkinje cell ensembles in multi-joint movement control. Curr Biol 25(9):1157–1165. https://doi.org/10.1016/j.cub.2015.03.009
Zhao Y, Kwan KM, Mailloux CM, Lee WK, Grinberg A, Wurst W, Behringer RR, Westphal H (2007) LIM-homeodomain proteins Lhx1 and Lhx5, and their cofactor Ldb1, control Purkinje cell differentiation in the developing cerebellum. Proc Natl Acad Sci USA 104(32):13182–13186. https://doi.org/10.1073/pnas.0705464104
Lui NC, Tam WY, Gao C, Huang JD, Wang CC, Jiang L, Yung WH, Kwan KM (2017) Lhx1/5 control dendritogenesis and spine morphogenesis of Purkinje cells via regulation of Espin. Nat Commun 8:15079. https://doi.org/10.1038/ncomms15079
Bottini AR, Gatti RA, Wirenfeldt M, Vinters HV (2012) Heterotopic Purkinje cells in ataxia-telangiectasia. Neuropathology 32(1):23–29. https://doi.org/10.1111/j.1440-1789.2011.01210.x
Yang Q, Hashizume Y, Yoshida M, Wang Y, Goto Y, Mitsuma N, Ishikawa K, Mizusawa H (2000) Morphological Purkinje cell changes in spinocerebellar ataxia type 6. Acta Neuropathol 100(4):371–376. https://doi.org/10.1007/s004010000201
Koeppen AH (1991) The Purkinje cell and its afferents in human hereditary ataxia. J Neuropathol Exp Neurol 50(4):505–514. https://doi.org/10.1097/00005072-199107000-00010
Wu Z, Sun F, Li Z, Liu M, Tian X, Guo D, Wei P, Shan Y et al. (2020) Electrical stimulation of the lateral cerebellar nucleus promotes neurogenesis in rats after motor cortical ischemia. Sci Rep 10(1):16563. https://doi.org/10.1038/s41598-020-73332-5
Cury RG, Franca C, Barbosa ER, Galhardoni R, Lepski G, Teixeira MJ, Ciampi de Andrade D (2019) Dentate nucleus stimulation in a patient with cerebellar ataxia and tremor after cerebellar stroke: a long-term follow-up. Parkinsonism Relat Disord 60:173–175. https://doi.org/10.1016/j.parkreldis.2018.10.001
Cooperrider J, Furmaga H, Plow E, Park HJ, Chen Z, Kidd G, Baker KB, Gale JT, et al. (2014) Chronic deep cerebellar stimulation promotes long-term potentiation, microstructural plasticity, and reorganization of perilesional cortical representation in a rodent model. J Neurosci 34(27):9040–9050. https://doi.org/10.1523/JNEUROSCI.0953-14.2014
Shah AM, Ishizaka S, Cheng MY, Wang EH, Bautista AR, Levy S, Smerin D, Sun G, et al. (2017) Optogenetic neuronal stimulation of the lateral cerebellar nucleus promotes persistent functional recovery after stroke. Sci Rep 7:46612. https://doi.org/10.1038/srep46612
Park HJ, Furmaga H, Cooperrider J, Gale JT, Baker KB, Machado AG (2015) Modulation of cortical motor evoked potential after stroke during electrical stimulation of the lateral cerebellar nucleus. Brain Stimul 8(6):1043–1048. https://doi.org/10.1016/j.brs.2015.06.020
Chan HH, Cooperrider J, Chen Z, Gale JT, Baker KB, Wathen CA, Modic CR, Park HJ, et al. (2018) Lateral cerebellar nucleus stimulation has selective effects on glutamatergic and GABAergic perilesional neurogenesis after Cortical Ischemia in the Rodent Model. Neurosurgery 83(5):1057–1067. https://doi.org/10.1093/neuros/nyx473
White JJ, Sillitoe RV (2017) Genetic silencing of olivocerebellar synapses causes dystonia-like behaviour in mice. Nat Commun 8:14912. https://doi.org/10.1038/ncomms14912
Mitoma H, Adhikari K, Aeschlimann D, Chattopadhyay P, Hadjivassiliou M, Hampe CS, Honnorat J, Joubert B, et al. (2016) Consensus Paper: Neuroimmune mechanisms of cerebellar ataxias. Cerebellum 15(2):213–232. https://doi.org/10.1007/s12311-015-0664-x
Hadjivassiliou M, Boscolo S, Tongiorgi E, Grunewald RA, Sharrack B, Sanders DS, Woodroofe N, Davies-Jones GA (2008) Cerebellar ataxia as a possible organ-specific autoimmune disease. Mov Disord 23(10):1370–1377. https://doi.org/10.1002/mds.22129
Mitoma H, Honnorat J, Yamaguchi K, Manto M (2020) Fundamental mechanisms of autoantibody-induced impairments on ion channels and synapses in immune-mediated cerebellar ataxias. Int J Mol Sci 21 (14). https://doi.org/10.3390/ijms21144936
Jarius S, Steinmeyer F, Knobel A, Streitberger K, Hotter B, Horn S, Heuer H, Schreiber SJ, et al. (2013) GABAB receptor antibodies in paraneoplastic cerebellar ataxia. J Neuroimmunol 256(1–2):94–96. https://doi.org/10.1016/j.jneuroim.2012.12.006
Chen Y, Zhu G, Liu D, Zhang X, Liu Y, Yuan T, Du T, Zhang J (2020) Subthalamic nucleus deep brain stimulation suppresses neuroinflammation by Fractalkine pathway in Parkinson’s disease rat model. Brain Behav Immun 90:16–25. https://doi.org/10.1016/j.bbi.2020.07.035
Dandekar MP, Saxena A, Scaini G, Shin JH, Migut A, Giridharan VV, Zhou Y, Barichello T, et al. (2019) Medial forebrain bundle deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: importance of BDNF and inflammatory cytokines. Mol Neurobiol 56(6):4364–4380. https://doi.org/10.1007/s12035-018-1381-5
Kwan KM, Behringer RR (2002) Conditional inactivation of Lim1 function. Genesis 32(2):118–120
Tam WY, Behringer R, Kwan KM (2011) Redundant functions of LIM-homeodomain transcription factors Lhx1 and Lhx5 on postnatal development of cerebellar Purkinje neurons in the mouse. Dev Biol 356(1):158–159. https://doi.org/10.1016/j.ydbio.2011.05.587
Scholle HC, Jinnah HA, Arnold D, Biedermann FH, Faenger B, Grassme R, Hess EJ, Schumann NP (2010) Kinematic and electromyographic tools for characterizing movement disorders in mice. Mov Disord 25(3):265–274. https://doi.org/10.1002/mds.22933
Brown AM, White JJ, van der Heijden ME, Zhou J, Lin T, Sillitoe RV (2020) Purkinje cell misfiring generates high-amplitude action tremors that are corrected by cerebellar deep brain stimulation. Elife 9. https://doi.org/10.7554/eLife.51928
Kumar G, Au NPB, Lei ENY, Mak YL, Chan LLH, Lam MHW, Chan LL, Lam PKS, et al. (2017) Acute exposure to pacific ciguatoxin reduces electroencephalogram activity and disrupts neurotransmitter metabolic pathways in motor cortex. Mol Neurobiol 54(7):5590–5603. https://doi.org/10.1007/s12035-016-0093-y
Asthana P, Zhang G, Sheikh KA, Him Eddie Ma C (2021) Heat shock protein is a key therapeutic target for nerve repair in autoimmune peripheral neuropathy and severe peripheral nerve injury. Brain Behav Immun 91:48–64. https://doi.org/10.1016/j.bbi.2020.08.020
Jayabal S, Ljungberg L, Watt AJ (2017) Transient cerebellar alterations during development prior to obvious motor phenotype in a mouse model of spinocerebellar ataxia type 6. J Physiol 595(3):949–966. https://doi.org/10.1113/JP273184
Dell’Orco JM, Pulst SM, Shakkottai VG (2017) Potassium channel dysfunction underlies Purkinje neuron spiking abnormalities in spinocerebellar ataxia type 2. Hum Mol Genet 26(20):3935–3945. https://doi.org/10.1093/hmg/ddx281
Fremont R, Calderon DP, Maleki S, Khodakhah K (2014) Abnormal high-frequency burst firing of cerebellar neurons in rapid-onset dystonia-parkinsonism. J Neurosci 34(35):11723–11732. https://doi.org/10.1523/JNEUROSCI.1409-14.2014
Servais L, Hourez R, Bearzatto B, Gall D, Schiffmann SN, Cheron G (2007) Purkinje cell dysfunction and alteration of long-term synaptic plasticity in fetal alcohol syndrome. Proc Natl Acad Sci U S A 104(23):9858–9863. https://doi.org/10.1073/pnas.0607037104
Anderson CJ, Figueroa KP, Dorval AD, Pulst SM (2019) Deep cerebellar stimulation reduces ataxic motor symptoms in the shaker rat. Ann Neurol 85(5):681–690. https://doi.org/10.1002/ana.25464
Teixeira MJ, Cury RG, Galhardoni R, Barboza VR, Brunoni AR, Alho E, Lepski G, Ciampi de Andrade D (2015) Deep brain stimulation of the dentate nucleus improves cerebellar ataxia after cerebellar stroke. Neurology 85(23):2075–2076. https://doi.org/10.1212/WNL.0000000000002204
Dayal V, Limousin P, Foltynie T (2017) Subthalamic nucleus deep brain stimulation in Parkinson’s disease: the effect of varying stimulation parameters. J Parkinsons Dis 7(2):235–245. https://doi.org/10.3233/JPD-171077
Milosevic L, Kalia SK, Hodaie M, Lozano A, Popovic MR, Hutchison W (2019) Subthalamic suppression defines therapeutic threshold of deep brain stimulation in Parkinson’s disease. J Neurol, Neurosurg & Psychiatry:jnnp-2019–321140. https://doi.org/10.1136/jnnp-2019-321140
Wilkes BJ, Wagle Shukla A, Casamento-Moran A, Hess CW, Christou EA, Okun MS, Vaillancourt DE (2020) Effects of ventral intermediate nucleus deep brain stimulation across multiple effectors in essential tremor. Clin Neurophysiol 131(1):167–176. https://doi.org/10.1016/j.clinph.2019.10.019
Kemp KC, Cerminara N, Hares K, Redondo J, Cook AJ, Haynes HR, Burton BR, Pook M, et al. (2017) Cytokine therapy-mediated neuroprotection in a Friedreich’s ataxia mouse model. Ann Neurol 81(2):212–226. https://doi.org/10.1002/ana.24846
Evert BO, Vogt IR, Kindermann C, Ozimek L, de Vos RA, Brunt ER, Schmitt I, Klockgether T, et al. (2001) Inflammatory genes are upregulated in expanded ataxin-3-expressing cell lines and spinocerebellar ataxia type 3 brains. J Neurosci 21(15):5389–5396
Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T (2012) AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther 19(7):724–733. https://doi.org/10.1038/gt.2011.126
Clausen BH, Lambertsen KL, Dagnaes-Hansen F, Babcock AA, von Linstow CU, Meldgaard M, Kristensen BW, Deierborg T, et al. (2016) Cell therapy centered on IL-1Ra is neuroprotective in experimental stroke. Acta Neuropathol 131(5):775–791. https://doi.org/10.1007/s00401-016-1541-5
Salmeron KE, Maniskas ME, Edwards DN, Wong R, Rajkovic I, Trout A, Rahman AA, Hamilton S, Fraser JF, et al. (2019) Interleukin 1 alpha administration is neuroprotective and neuro-restorative following experimental ischemic stroke. J Neuroinflammation 16(1):222. https://doi.org/10.1186/s12974-019-1599-9
Kelso ML, Elliott BR, Haverland NA, Mosley RL, Gendelman HE (2015) Granulocyte-macrophage colony stimulating factor exerts protective and immunomodulatory effects in cortical trauma. J Neuroimmunol 278:162–173. https://doi.org/10.1016/j.jneuroim.2014.11.002
Chen X, Zhang J, Song Y, Yang P, Yang Y, Huang Z, Wang K (2020) Deficiency of anti-inflammatory cytokine IL-4 leads to neural hyperexcitability and aggravates cerebral ischemia-reperfusion injury. Acta Pharm Sin B 10(9):1634–1645. https://doi.org/10.1016/j.apsb.2020.05.002
Rossi S, Mancino R, Bergami A, Mori F, Castelli M, De Chiara V, Studer V, Mataluni G, et al. (2011) Potential role of IL-13 in neuroprotection and cortical excitability regulation in multiple sclerosis. Mult Scler 17(11):1301–1312. https://doi.org/10.1177/1352458511410342
Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, et al. (2003) Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 349(20):1925–1934. https://doi.org/10.1056/NEJMoa035275
Johnson MD, Miocinovic S, McIntyre CC, Vitek JL (2008) Mechanisms and targets of deep brain stimulation in movement disorders. Neurotherapeutics 5(2):294–308. https://doi.org/10.1016/j.nurt.2008.01.010
Brown P, Mazzone P, Oliviero A, Altibrandi MG, Pilato F, Tonali PA, Di Lazzaro V (2004) Effects of stimulation of the subthalamic area on oscillatory pallidal activity in Parkinson’s disease. Exp Neurol 188(2):480–490. https://doi.org/10.1016/j.expneurol.2004.05.009
Kuhn AA, Kempf F, Brucke C, Gaynor Doyle L, Martinez-Torres I, Pogosyan A, Trottenberg T, et al. (2008) High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci 28(24):6165–6173. https://doi.org/10.1523/JNEUROSCI.0282-08.2008
Boraud T, Bezard E, Bioulac B, Gross C (1996) High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neurosci Lett 215(1):17–20. https://doi.org/10.1016/s0304-3940(96)12943-8
Dostrovsky JO, Levy R, Wu JP, Hutchison WD, Tasker RR, Lozano AM (2000) Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol 84(1):570–574. https://doi.org/10.1152/jn.2000.84.1.570
Tabata T, Kano M (2006) GABA(B) receptor-mediated modulation of glutamate signaling in cerebellar Purkinje cells. Cerebellum 5(2):127–133. https://doi.org/10.1080/14734220600788911
Ben Smail D, Jacq C, Denys P, Bussel B (2005) Intrathecal baclofen in the treatment of painful, disabling spasms in Friedreich’s ataxia. Mov Disor: Off J Mov Disor Soc 20(6):758–759
Miterko LN, Lin T, Zhou J, van der Heijden ME, Beckinghausen J, White JJ, Sillitoe RV (2021) Neuromodulation of the cerebellum rescues movement in a mouse model of ataxia. Nat Commun 12(1):1295. https://doi.org/10.1038/s41467-021-21417-8
Ertzgaard P, Campo C, Calabrese A (2017) Efficacy and safety of oral baclofen in the management of spasticity: a rationale for intrathecal baclofen. J Rehabil Med 49(3):193–203. https://doi.org/10.2340/16501977-2211
Bresolin N, Zucca C, Pecori A (2009) Efficacy and tolerability of eperisone and baclofen in spastic palsy: a double-blind randomized trial. Adv Ther 26(5):563–573. https://doi.org/10.1007/s12325-009-0031-8
Wingeier B, Tcheng T, Koop MM, Hill BC, Heit G, Bronte-Stewart HM (2006) Intra-operative STN DBS attenuates the prominent beta rhythm in the STN in Parkinson’s disease. Exp Neurol 197(1):244–251. https://doi.org/10.1016/j.expneurol.2005.09.016
Cooper SE, McIntyre CC, Fernandez HH, Vitek JL (2013) Association of deep brain stimulation washout effects with Parkinson disease duration. JAMA Neurol 70(1):95–99. https://doi.org/10.1001/jamaneurol.2013.581
Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ (2003) How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 60(1):78–81. https://doi.org/10.1212/wnl.60.1.78
Kahan J, Urner M, Moran R, Flandin G, Marreiros A, Mancini L, White M, Thornton J, et al. (2014) Resting state functional MRI in Parkinson’s disease: the impact of deep brain stimulation on “effective” connectivity. Brain 137(Pt 4):1130–1144. https://doi.org/10.1093/brain/awu027
Khaindrava V, Salin P, Melon C, Ugrumov M, Kerkerian-Le-Goff L, Daszuta A (2011) High frequency stimulation of the subthalamic nucleus impacts adult neurogenesis in a rat model of Parkinson’s disease. Neurobiol Dis 42(3):284–291. https://doi.org/10.1016/j.nbd.2011.01.018
Bergman H, Wichmann T, DeLong MR (1990) Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249(4975):1436–1438. https://doi.org/10.1126/science.2402638
Ashkan K, Rogers P, Bergman H, Ughratdar I (2017) Insights into the mechanisms of deep brain stimulation. Nat Rev Neurol 13(9):548–554. https://doi.org/10.1038/nrneurol.2017.105
Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, Matthews K, McIntyre CC, et al. (2019) Deep brain stimulation: current challenges and future directions. Nat Rev Neurol 15(3):148–160. https://doi.org/10.1038/s41582-018-0128-2
K SR, Rubakhin SS, Szucs A, Hughes TK, Stefano GB (1997) Opposite effects of interleukin-2 and interleukin-4 on GABA-induced inward currents of dialysed Lymnaea neurons. Gen Pharmacol 29 (1):73-7. https://doi.org/10.1016/s0306-3623(96)00527-7
Sloane E, Ledeboer A, Seibert W, Coats B, van Strien M, Maier SF, Johnson KW, Chavez R, et al. (2009) Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behav Immun 23(1):92–100. https://doi.org/10.1016/j.bbi.2008.09.004
Dinarello CA (1996) Biologic basis for interleukin-1 in disease. Blood 87(6):2095–2147
Helmy A, Guilfoyle MR, Carpenter KL, Pickard JD, Menon DK, Hutchinson PJ (2014) Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: a phase II randomized control trial. J Cereb Blood Flow Metab 34(5):845–851. https://doi.org/10.1038/jcbfm.2014.23
Funding
This work is supported in part by the Innovation and Technology Commission of the Hong Kong Special Administrative Region Government (ITS/151/17 and ITS/168/19FP) and The Health and Medical Research Fund (HMRF), Food and Health Bureau, and Hong Kong Special Administrative Region Government (07181356) award to Chi Him Eddie Ma.
Author information
Authors and Affiliations
Contributions
G. K. performed the surgery, in vivo animal behavioral assessments, in vivo recordings, and electrophysiology data analysis. P. A. maintained the Lhx1/5 and Pcp2-Cre mouse colonies and genotyping, and performed the cytokine array analysis. W. H. Y. and C. T. provided technical support and advice on DBS. K. M. K. provided the Lhx1/5 ataxia mice and genotyping protocol. C. H. E. M. conceived the project, designed the study, and wrote the manuscript with inputs from all authors. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, G., Asthana, P., Yung, W.H. et al. Deep Brain Stimulation of the Interposed Nucleus Reverses Motor Deficits and Stimulates Production of Anti-inflammatory Cytokines in Ataxia Mice. Mol Neurobiol 59, 4578–4592 (2022). https://doi.org/10.1007/s12035-022-02872-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02872-w