Abstract
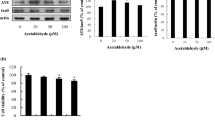
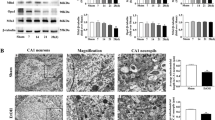
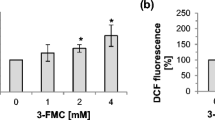
Overconsumption of alcohol damages brain tissue and causes cognitive dysfunction. It has been suggested that the neurotoxicity caused by excessive alcohol consumption is largely mediated by acetaldehyde, the most toxic metabolite of ethanol. Evidence shows that acetaldehyde impairs mitochondrial function and induces cytotoxicity of neuronal cells; however, the exact mechanisms are not fully understood. The aim of this study was to investigate the role of mitophagy in acetaldehyde-induced cytotoxicity. It was found that acetaldehyde treatment induced mitophagic responses and caused cytotoxicity in SH-SY5Y cells. The levels of light chain 3 (LC3)-II, Beclin1, autophagy-related protein (Atg) 5 and Atg16L1, PTEN-induced putative kinase (PINK)1, and Parkin were significantly elevated, while the level of p62 was reduced in acetaldehyde-treated cells. Acetaldehyde also promoted the accumulation of PINK1 and Parkin on mitochondria and caused a remarkable decrease of mitochondrial mass. Treatment with autophagy inhibitors prevented the decline of mitochondrial mass and alleviated the cytotoxicity induced by acetaldehyde, suggesting that overactive mitophagy might be an important mechanism contributing to acetaldehyde-induced cytotoxicity. Antioxidant N-acetyl-L-cysteine significantly attenuated the mitophagic responses and alleviated the cytotoxicity induced by acetaldehyde, indicating that oxidative stress was a major mediator of the excessive mitophagy induced by acetaldehyde. Taken together, these findings provided new insights into the role of mitophagy and oxidative stress in acetaldehyde-induced cytotoxicity.









Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Zhang K, Li Z, Jaiswal M, Bayat V, Xiong B, Sandoval H, Charng WL, David G, Haueter C, Yamamoto S, Graham BH, Bellen HJ (2013) The C8ORF38 homologue sicily is a cytosolic chaperone for a mitochondrial complex I subunit. J Cell Biol 200(6):807–820. https://doi.org/10.1083/jcb.201208033
Wang ZT, Lu MH, Zhang Y, Ji WL, Lei L, Wang W, Fang LP, Wang LW, Yu F, Wang J, Li ZY, Wang JR, Wang TH, Dou F, Wang QW, Wang XL, Li S, Ma QH, Xu RX (2019) Disrupted-in-schizophrenia-1 protects synaptic plasticity in a transgenic mouse model of Alzheimer’s disease as a mitophagy receptor. Aging Cell 18(1):e12860. https://doi.org/10.1111/acel.12860
Hsieh CH, Shaltouki A, Gonzalez AE, Bettencourt da Cruz A, Burbulla LF, St Lawrence E, Schule B, Krainc D, Palmer TD, Wang X (2016) Functional impairment in miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson’s disease. Cell Stem Cell 19(6):709–724. https://doi.org/10.1016/j.stem.2016.08.002
Shefa U, Jeong NY, Song IO, Chung HJ, Kim D, Jung J, Huh Y (2019) Mitophagy links oxidative stress conditions and neurodegenerative diseases. Neural Regen Res 14(5):749–756. https://doi.org/10.4103/1673-5374.249218
Lou G, Palikaras K, Lautrup S, Scheibye-Knudsen M, Tavernarakis N, Fang EF (2019) Mitophagy and neuroprotection. Trends Mol Med:30176–30188. https://doi.org/10.1016/j.molmed.2019.07.002
Johnson-Lyles DN, Peifley K, Lockett S, Neun BW, Hansen M, Clogston J, Stern ST, McNeil SE (2010) Fullerenol cytotoxicity in kidney cells is associated with cytoskeleton disruption, autophagic vacuole accumulation, and mitochondrial dysfunction. Toxicol Appl Pharmacol 248(3):249–258. https://doi.org/10.1016/j.taap.2010.08.008
Cherra SJ 3rd, Steer E, Gusdon AM, Kiselyov K, Chu CT (2013) Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. Am J Pathol 182(2):474–484. https://doi.org/10.1016/j.ajpath.2012.10.027
Su SH, Wu YF, Wang DP, Hai J (2018) Inhibition of excessive autophagy and mitophagy mediates neuroprotective effects of URB597 against chronic cerebral hypoperfusion. Cell Death Disease 9(7):733–747. https://doi.org/10.1038/s41419-018-0755-y
Nakatsu Y, Kotake Y, Takai N, Ohta S (2010) Involvement of autophagy via mammalian target of rapamycin (mTOR) inhibition in tributyltin-induced neuronal cell death. J Toxicol Sci 35(2):245–251. https://doi.org/10.2131/jts.35.245
Wang DD, Jin MF, Zhao DJ, Ni H (2019) Reduction of mitophagy-related oxidative stress and preservation of mitochondria function using melatonin therapy in an HT22 hippocampal neuronal cell model of glutamate-induced excitotoxicity. Front Endocrinol (Lausanne) 10:550. https://doi.org/10.3389/fendo.2019.00550
Yang JY, Xue X, Tian H, Wang XX, Dong YX, Wang F, Zhao YN, Yao XC, Cui W, Wu CF (2014) Role of microglia in ethanol-induced neurodegenerative disease: Pathological and behavioral dysfunction at different developmental stages. Pharmacol Ther 144(3):321–337. https://doi.org/10.1016/j.pharmthera.2014.07.002
Yan T, Zhao Y, Zhang X, Lin X (2016) Astaxanthin inhibits acetaldehyde-induced cytotoxicity in SH-SY5Y cells by modulating Akt/CREB and p38MAPK/ERK signaling pathways. Mar Drugs 14(3):56–68. https://doi.org/10.3390/md14030056
Haorah J, Floreani NA, Knipe B, Persidsky Y (2011) Stabilization of superoxide dismutase by acetyl-l-carnitine in human brain endothelium during alcohol exposure: novel protective approach. Free Radic Biol Med 51(8):1601–1609. https://doi.org/10.1016/j.freeradbiomed.2011.06.020
Cui J, Liu Y, Chang X, Gou W, Zhou X, Liu Z, Li Z, Wu Y, Zuo D (2019) Acetaldehyde induces neurotoxicity in vitro via oxidative stress- and Ca(2+) imbalance-mediated endoplasmic reticulum stress. Oxid Med Cell Longev 2019:1–8. https://doi.org/10.1155/2019/2593742
Tokuda K, Izumi Y, Zorumski CF (2013) Locally-generated acetaldehyde is involved in ethanol-mediated LTP inhibition in the hippocampus. Neurosci Lett 537:40–43. https://doi.org/10.1016/j.neulet.2013.01.018
Peng GS, Yin SJ (2008) Effect of the allelic variants of aldehyde dehydrogenase ALDH2*2 and alcohol dehydrogenase ADH1B*2 on blood acetaldehyde concentrations. Hum Genomics 3(2):121–127. https://doi.org/10.1186/1479-7364-3-2-121
Chen Y, Lu R, Peng G, Wang M, Wang H, Ko H, Chang Y, Lu J, Li T, Yin S (2006) Alcohol metabolism and cardiovascular response in an alcoholic patient homozygous for the ALDH2*2 variant gene allele. Alcohol Clin Exp Res 23(12):1853–1860. https://doi.org/10.1097/00000374-199912000-00001
Dezest M, Le Bechec M, Chavatte L, Desauziers V, Chaput B, Grolleau JL, Descargues P, Nizard C, Schnebert S, Lacombe S, Bulteau AL (2017) Oxidative damage and impairment of protein quality control systems in keratinocytes exposed to a volatile organic compounds cocktail. Sci Rep 7(1):10707. https://doi.org/10.1038/s41598-017-11088-1
Brandt M, Garlapati V, Oelze M, Sotiriou E, Knorr M, Kroller-Schon S, Kossmann S, Schonfelder T, Morawietz H, Schulz E, Schultheiss HP, Daiber A, Munzel T, Wenzel P (2016) NOX2 amplifies acetaldehyde-mediated cardiomyocyte mitochondrial dysfunction in alcoholic cardiomyopathy. Sci Rep 6:32554. https://doi.org/10.1038/srep32554
Yan T, Zhao Y (2020) Acetaldehyde induces phosphorylation of dynamin-related protein 1 and mitochondrial dysfunction via elevating intracellular ROS and Ca(2+) levels. Redox Biol 28:101381. https://doi.org/10.1016/j.redox.2019.101381
Joshi AU, Van Wassenhove LD, Logas KR, Minhas PS, Andreasson KI, Weinberg KI, Chen CH, Mochly-Rosen D (2019) Aldehyde dehydrogenase 2 activity and aldehydic load contribute to neuroinflammation and Alzheimer’s disease related pathology. Acta Neuropathol Commun 7(1):190. https://doi.org/10.1186/s40478-019-0839-7
Guzman JN, Ilijic E, Yang B, Sanchez-Padilla J, Wokosin D, Galtieri D, Kondapalli J, Schumacker PT, Surmeier DJ (2018) Systemic isradipine treatment diminishes calcium-dependent mitochondrial oxidant stress. J Clin Invest 128(6):2266–2280. https://doi.org/10.1172/JCI95898
Guo R, Hu N, Kandadi MR, Ren J (2012) Facilitated ethanol metabolism promotes cardiomyocyte contractile dysfunction through autophagy in murine hearts. Autophagy 8(4):593–608. https://doi.org/10.4161/auto.18997
Thapaliya S, Runkana A, McMullen MR, Nagy LE, McDonald C, Naga Prasad SV, Dasarathy S (2014) Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy 10(4):677–690. https://doi.org/10.4161/auto.27918
Girault V, Gilard V, Marguet F, Lesueur C, Hauchecorne M, Ramdani Y, Laquerriere A, Marret S, Jegou S, Gonzalez BJ, Brasse-Lagnel C, Bekri S (2017) Prenatal alcohol exposure impairs autophagy in neonatal brain cortical microvessels. Cell Death Dis 8(2):e2610. https://doi.org/10.1038/cddis.2017.29
Fang EF, Waltz TB, Kassahun H, Lu Q, Kerr JS, Morevati M, Fivenson EM, Wollman BN, Marosi K, Wilson MA, Iser WB, Eckley DM, Zhang Y, Lehrmann E, Goldberg IG, Scheibye-Knudsen M, Mattson MP, Nilsen H, Bohr VA, Becker KG (2017) Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway. Sci Rep 7:46208. https://doi.org/10.1038/srep46208
Villalta JI, Galli S, Iacaruso MF, Antico Arciuch VG, Poderoso JJ, Jares-Erijman EA, Pietrasanta LI (2011) New algorithm to determine true colocalization in combination with image restoration and time-lapse confocal microscopy to MAP kinases in mitochondria. PLoS ONE 6(4):e19031. https://doi.org/10.1371/journal.pone.0019031
Rodriguez ME, Azizuddin K, Zhang P, Chiu SM, Lam M, Kenney ME, Burda C, Oleinick NL (2008) Targeting of mitochondria by 10-N-alkyl acridine orange analogues: role of alkyl chain length in determining cellular uptake and localization. Mitochondrion 8(3):237–246. https://doi.org/10.1016/j.mito.2008.04.003
Labuschagne CF, Cheung EC, Blagih J, Domart MC, Vousden KH (2019) Cell clustering promotes a metabolic switch that supports metastatic colonization. Cell Metab 30 (4):720–734 e725. https://doi.org/10.1016/j.cmet.2019.07.014
Rai Y, Pathak R, Kumari N, Sah DK, Pandey S, Kalra N, Soni R, Dwarakanath BS, Bhatt AN (2018) Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci Rep 8(1):1531–1546. https://doi.org/10.1038/s41598-018-19930-w
Twig G, Elorza A, Molina AJ, Mohamed H (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27:433-446. 10.1038/
Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Hoke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H (2014) Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J 33 (23):2798–2813. https://doi.org/10.15252/embj.201488658
Shim MS, Nettesheim A, Hirt J, Liton PB (2020) The autophagic protein LC3 translocates to the nucleus and localizes in the nucleolus associated to NUFIP1 in response to cyclic mechanical stress. Autophagy 16(7):1248–1261. https://doi.org/10.1080/15548627.2019.1662584
Leidal AM, Huang HH, Marsh T, Solvik T, Zhang D, Ye J, Kai F, Goldsmith J, Liu JY, Huang YH, Monkkonen T, Vlahakis A, Huang EJ, Goodarzi H, Yu L, Wiita AP, Debnath J (2020) The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat Cell Biol 22(2):187–199. https://doi.org/10.1038/s41556-019-0450-y
Tan VP, Smith JM, Tu M, Yu J, Ding E, Miyamoto S (2019) Dissociation of mitochondrial HK-II elicits mitophagy and confers cardioprotection against ischemia. Cell Death Dis 10:730–745. https://doi.org/10.1038/s41419-019-1965-7
Li XJ, Zhang YY, Fu YH, Zhang H, Li HX, Li QF, Li HL, Tan RK, Jiang CX, Jiang W, Li ZX, Luo C, Lu BX, Dang YJ (2021) Gossypol, a novel modulator of VCP, induces autophagic degradation of mutant huntingtin by promoting the formation of VCP/p97-LC3-mHTT complex. Acta Pharmacol Sin. https://doi.org/10.1038/s41401-020-00605-0
Liu S, Mok BW, Deng S, Liu H, Wang P, Song W, Chen P, Huang X, Zheng M, Lau SY, Cremin CJ, Tam CY, Li B, Jiang L, Chen Y, Yuen KY, Chen H (2021) Mammalian cells use the autophagy process to restrict avian influenza virus replication. Cell Rep 35(10):109213. https://doi.org/10.1016/j.celrep.2021.109213
Ragazzoni Y, Desideri M, Gabellini C, De Luca T, Carradori S, Secci D, Nescatelli R, Candiloro A, Condello M, Meschini S, Del Bufalo D, Trisciuoglio D (2013) The thiazole derivative CPTH6 impairs autophagy. Cell Death Dis 4:e524. https://doi.org/10.1038/cddis.2013.53
Choubey V, Zeb A, Kaasik A (2021) Molecular mechanisms and regulation of mammalian mitophagy. Cells 11(1):38. https://doi.org/10.3390/cells11010038
Lin MW, Lin CC, Chen YH, Yang HB, Hung SY (2019) Celastrol inhibits dopaminergic neuronal death of Parkinson’s disease through activating mitophagy. Antioxidants (Basel) 9(1):37–54. https://doi.org/10.3390/antiox9010037
Pandey R, Bakay M, Hain HS, Strenkowski B, Elsaqa BZB, Roizen JD, Kushner JA, Orange JS, Hakonarson H (2018) CLEC16A regulates splenocyte and NK cell function in part through MEK signaling. PLoS ONE 13(9):e0203952. https://doi.org/10.1371/journal.pone.0203952
Ye X, Sun X, Starovoytov V, Cai Q (2015) Parkin-mediated mitophagy in mutant hAPP neurons and Alzheimer’s disease patient brains. Hum Mol Genet 24(10):2938–2951. https://doi.org/10.1093/hmg/ddv056
Nassif M, Valenzuela V, Rojas-Rivera D, Vidal R, Matus S, Castillo K, Fuentealba Y, Kroemer G, Levine B, Hetz C (2014) Pathogenic role of BECN1/Beclin 1 in the development of amyotrophic lateral sclerosis. Autophagy 10(7):1256–1271. https://doi.org/10.4161/auto.28784
Matoba K, Noda NN (2021). Structural catalog of core Atg proteins opens new era of autophagy research. J Biochem 169(5):517–525. https:// doi: https://doi.org/10.1093/jb/mvab017.
Kaufmann A, Beier V, Franquelim HG, Wollert T (2014) Molecular mechanism of autophagic membrane-scaffold assembly and disassembly. Cell 156(3):469–481. https://doi.org/10.1016/j.cell.2013.12.022
Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524(7565):309–314. https://doi.org/10.1038/nature14893
Shen M, Jiang Y, Guan Z, Cao Y, Sun SC, Liu H (2016) FSH protects mouse granulosa cells from oxidative damage by repressing mitophagy. Sci Rep 6:38090. https://doi.org/10.1038/srep38090
Ren Y, Li Y, Yan J, Ma M, Zhou D, Xue Z, Zhang Z, Liu H, Yang H, Jia L, Zhang L, Zhang Q, Mu S, Zhang R, Da Y (2017) Adiponectin modulates oxidative stress-induced mitophagy and protects C2C12 myoblasts against apoptosis. Sci Rep 7(1):3209–3221. https://doi.org/10.1038/s41598-017-03319-2
Lin W, Kang UJ (2008) Characterization of PINK1 processing, stability, and subcellular localization. J Neurochem 106(1):464–474. https://doi.org/10.1111/j.1471-4159.2008.05398.x
Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ (2010) PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8(1):e1000298. https://doi.org/10.1371/journal.pbio.1000298
Qi Y, Qiu Q, Gu X, Tian Y, Zhang Y (2016) ATM mediates spermidine-induced mitophagy via PINK1 and Parkin regulation in human fibroblasts. Sci Rep 6:24700. https://doi.org/10.1038/srep24700
Guo X, Sun X, Hu D, Wang YJ, Fujioka H, Vyas R, Chakrapani S, Joshi AU, Luo Y, Mochly-Rosen D, Qi X (2016) VCP recruitment to mitochondria causes mitophagy impairment and neurodegeneration in models of Huntington’s disease. Nat Commun 7:12646. https://doi.org/10.1038/ncomms12646
Qiao A, Wang K, Yuan YS, Guan Y (2016) Sirt3-mediated mitophagy protects tumor cells against apoptosis under hypoxia. Oncotarget 7 (28):43390–43399. https://doi.org/10.18632/oncotarget.9717
Salvi M, Kontro H, Cannino G, Rustin P, Dufour E, Kainulainen H (2015) DAPIT over-expression modulates glucose metabolism and cell behaviour in HEK293T cells. PLoS ONE 10(7):e0131990. https://doi.org/10.1371/journal.pone.0131990
Jeong S, Kim H, Song I-S, Noh S, Marquez J, Ko K, Rhee B, Kim N, Mishchenko N, Fedoreyev S, Stonik V, Han J (2014) Echinochrome a increases mitochondrial mass and function by modulating mitochondrial biogenesis regulatory genes. Mar Drugs 12(8):4602–4615. https://doi.org/10.3390/md12084602
Namba T (2019) BAP31 regulates mitochondrial function via interaction with Tom40 within ER-mitochondria contact sites. Sci Adv 5(6):1–12. https://doi.org/10.1126/sciadv.aaw1386
Kohsaka A, Das P, Hashimoto I, Nakao T, Deguchi Y, Gouraud SS, Waki H, Muragaki Y, Maeda M (2014) The circadian clock maintains cardiac function by regulating mitochondrial metabolism in mice. PLoS ONE 9(11):e112811. https://doi.org/10.1371/journal.pone.0112811
Vainshtein A, Desjardins EM, Armani A, Sandri M, Hood DA (2015) PGC-1alpha modulates denervation-induced mitophagy in skeletal muscle. Skelet Muscle 5:9. https://doi.org/10.1186/s13395-015-0033-y
Yin W, Signore AP, Iwai M, Cao G, Gao Y, Chen J (2008) Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke 39(11):3057–3063. https://doi.org/10.1161/strokeaha.108.520114
Hasnat M, Yuan Z, Naveed M, Khan A, Raza F, Xu D, Ullah A, Sun L, Zhang L, Jiang Z (2019) Drp1-associated mitochondrial dysfunction and mitochondrial autophagy: a novel mechanism in triptolide-induced hepatotoxicity. Cell Biol Toxicol 35(3):267–280. https://doi.org/10.1007/s10565-018-9447-8
Itoh K, Nakamura K, Iijima M, Sesaki H (2013) Mitochondrial dynamics in neurodegeneration. Trends Cell Biol 23(2):64–71. https://doi.org/10.1016/j.tcb.2012.10.006
Kim H, Lee JY, Park KJ, Kim WH, Roh GS (2016) A mitochondrial division inhibitor, Mdivi-1, inhibits mitochondrial fragmentation and attenuates kainic acid-induced hippocampal cell death. BMC Neurosci 17(1):33. https://doi.org/10.1186/s12868-016-0270-y
Apostolova N, Victor VM (2015) Molecular strategies for targeting antioxidants to mitochondria: therapeutic implications. Antioxid Redox Signal 22(8):686–729. https://doi.org/10.1089/ars.2014.5952
Tong M, Longato L, Nguyen Q-G, Chen WC, Spaisman A, de la Monte SM (2011) Acetaldehyde-mediated neurotoxicity: relevance to fetal alcohol spectrum disorders. Oxid Med Cell Longev 2011:1–13. https://doi.org/10.1155/2011/213286
Yamaguchi M, Noda NN, Yamamoto H, Shima T, Kumeta H, Kobashigawa Y, Akada R, Ohsumi Y, Inagaki F (2012) Structural insights into Atg10-mediated formation of the autophagy-essential Atg12-Atg5 conjugate. Structure 20(7):1244–1254. https://doi.org/10.1016/j.str.2012.04.018
Chen D, Fan W, Lu Y, Ding X, Chen S, Zhong Q (2012) A mammalian autophagosome maturation mechanism mediated by TECPR1 and the Atg12-Atg5 conjugate. Mol Cell 45(5):629–641. https://doi.org/10.1016/j.molcel.2011.12.036
Kumar D, Das B, Sen R, Kundu P, Manna A, Sarkar A, Chowdhury C, Chatterjee M, Das P (2015) Andrographolide analogue induces apoptosis and autophagy mediated cell death in U937 cells by inhibition of PI3K/Akt/mTOR pathway. PLoS ONE 10(10):e0139657. https://doi.org/10.1371/journal.pone.0139657
Button RW, Roberts SL, Willis TL, Hanemann CO, Luo S (2017) Accumulation of autophagosomes confers cytotoxicity. J Biol Chem 292(33):13599–13614. https://doi.org/10.1074/jbc.M117.782276
Rakovic A, Shurkewitsch K, Seibler P, Grunewald A, Zanon A, Hagenah J, Krainc D, Klein C (2013) Phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1)-dependent ubiquitination of endogenous Parkin attenuates mitophagy: study in human primary fibroblasts and induced pluripotent stem cell-derived neurons. J Biol Chem 288(4):2223–2237. https://doi.org/10.1074/jbc.M112.391680
Chen S, Zhou L, Zhang Y, Leng Y (2014) Targeting SQSTM1_p62 induces cargo loading failure and converts autophagy to apoptosis via NBK/Bik. Mol Cell Biol 34(18):3435–3449. https://doi.org/10.1128/MCB.01383-13
Litwiniuk A, Domanska A, Chmielowska M, Martynska L, Bik W, Kalisz M (2020) The effects of alpha-linolenic acid on the secretory activity of astrocytes and beta amyloid-associated neurodegeneration in differentiated SH-SY5Y cells: alpha-linolenic acid protects the SH-SY5Y cells against beta amyloid toxicity. Oxid Med Cell Longev 2020:8908901. https://doi.org/10.1155/2020/8908901
Qi Y, Ma R, Li X, Lv S, Liu X, Abulikemu A, Zhao X, Li Y, Guo C, Sun Z (2020) Disturbed mitochondrial quality control involved in hepatocytotoxicity induced by silica nanoparticles. Nanoscale 12(24):13034–13045. https://doi.org/10.1039/d0nr01893g
Xu J, Wang L, Zhang L, Zheng F, Wang F, Leng J, Wang K, Heroux P, Shen HM, Wu Y, Xia D (2020) Mono-2-ethylhexyl phthalate drives progression of PINK1-parkin-mediated mitophagy via increasing mitochondrial ROS to exacerbate cytotoxicity. Redox Biol 38:101776. https://doi.org/10.1016/j.redox.2020.101776
Zhang Y, Ma Y, Xiao Y, Lu C, Xiao F (2020) Drp1-dependent mitochondrial fission contributes to Cr(VI)-induced mitophagy and hepatotoxicity. Ecotoxicol Environ Saf 203:110928. https://doi.org/10.1016/j.ecoenv.2020.110928
Chen N, Guo Z, Luo Z, Zheng F, Shao W, Yu G, Cai P, Wu S, Li H (2021) Drp1-mediated mitochondrial fission contributes to mitophagy in paraquat-induced neuronal cell damage. Environ Pollut 272:116413. https://doi.org/10.1016/j.envpol.2020.116413
Chen Y, Azad M, Gibson S (2009) Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ 16(7):1040–1052. https://doi.org/10.1038/cdd.2009.49
Knuppertz L, Warnsmann V, Hamann A, Grimm C, Osiewacz HD (2017) Stress-dependent opposing roles for mitophagy in aging of the ascomycete podospora anserina. Autophagy 13(6):1037–1052. https://doi.org/10.1080/15548627.2017.1303021
Han H, Chou CC, Li R, Liu J, Zhang L, Zhu W, Hu J, Yang B, Tian J (2018) Chalcomoracin is a potent anticancer agent acting through triggering oxidative stress via a mitophagy- and paraptosis-dependent mechanism. Sci Rep 8(1):9566–9580. https://doi.org/10.1038/s41598-018-27724-3
Funding
This work was supported by the National Natural Science Foundation of China [31371082], Natural Science Foundation of Shandong province (ZR2019MH048), Weihai Science and Technology Development Program, and research fund from Harbin Institute of Technology at Weihai [HIT(WH)Y200902].
Author information
Authors and Affiliations
Contributions
TTY contributed to part of the experiments, article writing, and data analyses; YZ supervised the experiments, revised the manuscript, and helped with the technical problems; ZYJ and JYC performed part of the experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, T., Zhao, Y., Jiang, Z. et al. Acetaldehyde Induces Cytotoxicity via Triggering Mitochondrial Dysfunction and Overactive Mitophagy. Mol Neurobiol 59, 3933–3946 (2022). https://doi.org/10.1007/s12035-022-02828-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02828-0