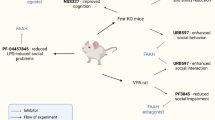
Abstract
Autism spectrum disorder (ASD) is a disease characterized by reduced social interaction and stereotypic behaviors and related to macroscopic volumetric changes in cerebellar and somatosensory cortices (SPP). Epidemiological and preclinical models have confirmed that a proinflammatory profile during fetal development increases ASD susceptibility after birth. Here, we aimed to globally identify the effect of maternal exposure to high-energy dense diets, which we refer to as cafeteria diet (CAF) on peripheral and central proinflammatory profiles, microglia reactivity, and volumetric brain changes related to assisting defective social interaction in the mice offspring. We found a sex-dependent effect of maternal exposure to CAF diet or inoculation of the dsARN mimetic Poly (I:C) on peripheral proinflammatory and social interaction in the offspring. Notably, maternal exposure to CAF diet impairs social interaction and favors an increase in anxiety in male but not female offspring. Also, CAF diet exposure or Poly (I:C) inoculation during fetal programming promote peripheral proinflammatory profile in the ASD-diagnosed male but not in females. Selectively, we found a robust accumulation of the monocyte chemoattractant protein-1 (MCP-1) in plasma of ASD-diagnosed males exposed to CAF during fetal development. Biological assessment of MCP-1 signaling in brain confirms that systemic injection of MCP-1-neutralizing antibody reestablished social interaction and blocked anxiety, accompanied by a reduction in cerebellar lobule X (CbX) volume and an increase volume of the primary somatosensory (SSP) cortex in male offspring. These data highlight the contribution of diet-dependent MCP-1 signaling on volumetric brain changes and microglia morphology promoting ASD-like behavior in male mice.








Similar content being viewed by others
Availability of Data and Materials
This study does not include material from living or dead patients. However, all the mice data and/or biological material will be made available under request.
References
Christensen DL, Baio J, Braun KVN, Bilder D, Charles J, Constantino JN et al (2016) Prevalence and characteristics of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 Sites, United States, 2012. MMWR Surveill Summ 65:1–23
Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J (2018) Autism spectrum disorder. Lancet 392:508–520
Mizuno Y, Kagitani-Shimono K, Jung M, Makita K, Takiguchi S, Fujisawa TX et al (2019) Structural brain abnormalities in children and adolescents with comorbid autism spectrum disorder and attention-deficit/hyperactivity disorder. Transl Psychiatry 9:332
Conti E, Retico A, Palumbo L, Spera G, Bosco P, Biagi L et al (2020) Autism spectrum disorder and childhood apraxia of speech: early language-related hallmarks across structural MRI study. J Pers Med 10:275
Smith REW, Avery JA, Wallace GL, Kenworthy L, Gotts SJ, Martin A. Sex differences in resting-state functional connectivity of the cerebellum in autism spectrum disorder. Front Hum Neurosci. 2019;13.
Li Y, Zhou Z, Chang C, Qian L, Li C, Xiao T et al (2019) Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry 19:399
Temur HO, Yurtsever I, Yesil G, Sharifov R, Yilmaz FT, Dundar TT et al (2019) Correlation between DTI findings and volume of corpus callosum in children with autism. Curr Med Imaging Former Curr Med Imaging Rev 15:895–899
Postema MC, van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M et al (2019) Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nat Commun 10:4958
Varghese M, Keshav N, Jacot-Descombes S, Warda T, Wicinski B, Dickstein DL et al (2017) Autism spectrum disorder: neuropathology and animal models. Acta Neuropathol 134:537–566
van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF et al (2018) Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: results from the ENIGMA ASD working group. Am J Psychiatry 175:359–369
Stoodley CJ, D’Mello AM, Ellegood J, Jakkamsetti V, Liu P, Nebel MB et al (2017) Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat Neurosci 20:1744–1751
Shin Y, Park A, Berrios J, Lafourcade M, Pascual LM, Soares N et al (2017) Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549(7673):482–487. https://doi.org/10.1038/nature23909 (Epub 2017 Sep 13)
Doenyas C (2018) Gut Microbiota, inflammation, and probiotics on neural development in autism spectrum disorder. Neuroscience 374:271–286
Sanders SJ (2015) First glimpses of the neurobiology of autism spectrum disorder. Curr Opin Genet Dev 33:80–92
Lombardo MV, Moon HM, Su J, Palmer TD, Courchesne E, Pramparo T (2018) Maternal immune activation dysregulation of the fetal brain transcriptome and relevance to the pathophysiology of autism spectrum disorder. Mol Psychiatry 23:1001–1013
Jiang H, Xu L, Shao L, Xia R, Yu Z, Ling Z, et al. Maternal infection during pregnancy and risk of autism spectrum disorders: a systematic review and meta-analysis. Brain Behav Immun. 2016. 2016. https://doi.org/10.1016/j.bbi.2016.06.005.
Mahic M, Mjaaland S, Bøvelstad HM, Gunnes N, Susser E, Bresnahan M et al (2017) Maternal immunoreactivity to herpes simplex virus 2 and risk of autism spectrum disorder in male offspring. MSphere 2:e00016-17
Careaga M, Murai T, Bauman MD. Maternal immune activation and autism spectrum disorder: from rodents to nonhuman and human primates. Biol Psychiatry. 2017.
Chen HJ, Antonson AM, Rajasekera TA, Patterson JM, Bailey MT, Gur TL. Prenatal stress causes intrauterine inflammation and serotonergic dysfunction, and long-term behavioral deficits through microbe- and CCL2-dependent mechanisms. Transl Psychiatry. 2020. 2020. https://doi.org/10.1038/s41398-020-00876-5.
Nadeem A, Ahmad SF, Bakheet SA, Al-Harbi NO, AL-Ayadhi LY, Attia SM, et al. Toll-like receptor 4 signaling is associated with upregulated NADPH oxidase expression in peripheral T cells of children with autism. Brain Behav Immun. 2017;61:146–154.
Nadeem A, Ahmad SF, Attia SM, Bakheet SA, Al-Harbi NO, AL-Ayadhi LY. Activation of IL-17 receptor leads to increased oxidative inflammation in peripheral monocytes of autistic children. Brain Behav Immun. 2018. 2018. https://doi.org/10.1016/j.bbi.2017.09.010.
Cruz-carrillo G, Montalvo-martínez L, Cárdenas-tueme M, Bernal-vega S, Maldonado-ruiz R, Reséndez-pérez D et al (2020) Fetal programming by methyl donors modulates central inflammation and prevents food addiction-like behavior in rats. 14:1–15
Bordeleau M, Lacabanne C, Fernández de Cossío L, Vernoux N, Savage JC, González-Ibáñez F, et al. Microglial and peripheral immune priming is partially sexually dimorphic in adolescent mouse offspring exposed to maternal high-fat diet. J Neuroinflammation. 2020;17:264.
Thompson MD, Derse A, LA Ferey J, Reid M, Xie Y, Christ M et al (2019) Transgenerational impact of maternal obesogenic diet on offspring bile acid homeostasis and nonalcoholic fatty liver disease. Am J Physiol Metab 316:E674–E686
Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010. 2010. https://doi.org/10.1096/fj.09-144014.
Maldonado-Ruiz R, Cárdenas-Tueme M, Montalvo-Martínez L, Vidaltamayo R, Garza-Ocañas L, Reséndez-Perez D et al (2019) Priming of hypothalamic ghrelin signaling and microglia activation exacerbate feeding in rats’ offspring following maternal overnutrition. Nutrients 11:1241
Trujillo-Villarreal LA, Romero-Díaz VJ, Marino-Martínez IA, Fuentes-Mera L, Ponce-Camacho MA, Devenyi GA et al (2021) Maternal cafeteria diet exposure primes depression-like behavior in the offspring evoking lower brain volume related to changes in synaptic terminals and gliosis. Transl Psychiatry 11:53
Maldonado-Ruiz R, Cárdenas-Tueme M, Montalvo-Martínez L, Vidaltamayo R, Garza-Ocañas L, Reséndez-Perez D, et al. Priming of hypothalamic ghrelin signaling and microglia activation exacerbate feeding in rats’ offspring following maternal overnutrition. Nutrients. 2019;11.
de la Garza A, Garza-Cuellar M, Silva-Hernandez I, Cardenas-Perez R, Reyes-Castro L, Zambrano E et al (2019) Maternal flavonoids intake reverts depression-like behaviour in rat female offspring. Nutrients 11:572
Camacho A, Montalvo-Martinez L, Cardenas-Perez RE, Fuentes-Mera L, Garza-Ocañas L (2017) Obesogenic diet intake during pregnancy programs aberrant synaptic plasticity and addiction-like behavior to a palatable food in offspring. Behav Brain Res 330:46–55
Cardenas-Perez RE, Fuentes-Mera L, de la Garza AL, Torre-Villalvazo I, Reyes-Castro LA, Rodriguez-Rocha H et al (2018) Maternal overnutrition by hypercaloric diets programs hypothalamic mitochondrial fusion and metabolic dysfunction in rat male offspring. Nutr Metab (Lond) 15:38
Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J Vis Exp. 2013. 2013. https://doi.org/10.3791/50978.
Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR. Assessment of social interaction behaviors. J Vis Exp. 2010.
Slimani H, Zhai Y, Yousif NG, Ao L, Zeng Q, Fullerton DA, et al. Enhanced monocyte chemoattractant protein-1 production in aging mice exaggerates cardiac depression during endotoxemia. Crit Care. 2014. 2014. https://doi.org/10.1186/s13054-014-0527-8.
Avants B, Epstein C, Grossman M, Gee JC (2008) Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 48(Suppl 2):1–6
Bird S, Klein E, Loper E. Natural Language Processing with Python. O´Really; 2009.
Lerch JP, Sled JG, Henkelman RM. Magnetic resonance neuroimaging. In: magnetic resonance neuroimaging, Methods in Molecular Biology. 2011. p. 349–61.
Chung MK, Worsley KJ, Paus T, Cherif C, Collins DL, Giedd JN et al (2001) A unified statistical approach to deformation-based morphometry. Neuroimage 14(3):595–606
Leow AD, Klunder AD, Jr. CRJ, Toga AW, Dale AM, Bernstein MA, et al. Longitudinal stability of MRI for mapping brain change using tensor-based morphometry. Neuroimage. 2007;31(2):627–40.
Franklin KBJ, Paxinos G. Molecular Psychiatry. 2007.
Haddad FL, Patel SV, Schmid S (2020) Maternal immune activation by poly I: C as a preclinical model for neurodevelopmental disorders: a focus on autism and schizophrenia. Neurosci Biobehav Rev 113:546–567
Saghazadeh A, Ataeinia B, Keynejad K, Abdolalizadeh A, Hirbod-Mobarakeh A, Rezaei N (2019) A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: Effects of age, gender, and latitude. J Psychiatr Res 115:90–102
Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, Guastella AJ (2015) Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatry 20:440–446
Xie J, Huang L, Li X, Li H, Zhou Y, Zhu H, et al. Immunological cytokine profiling identifies TNF-α as a key molecule dysregulated in autistic children. Oncotarget. 2017. 2017. https://doi.org/10.18632/oncotarget.19326.
Chen HJ, Antonson AM, Rajasekera TA, Patterson JM, Bailey MT, Gur TL (2020) Prenatal stress causes intrauterine inflammation and serotonergic dysfunction, and long-term behavioral deficits through microbe- and CCL2-dependent mechanisms. Transl Psychiatry 10:191
Shen Y, Ou Ji, Liu M, Shi L, Li Y, Xiao L, et al. Altered plasma levels of chemokines in autism and their association with social behaviors. Psychiatry Res. 2016;244:300–305.
Sarker G, Sun W, Rosenkranz D, Pelczar P, Opitz L, Efthymiou V et al (2019) Maternal overnutrition programs hedonic and metabolic phenotypes across generations through sperm tsRNAs. Proc Natl Acad Sci 116:10547–10556
Cinquina V, Calvigioni D, Farlik M, Halbritter F, Fife-Gernedl V, Shirran SL et al (2020) Life-long epigenetic programming of cortical architecture by maternal ‘Western’ diet during pregnancy. Mol Psychiatry 25:22–36
Thompson JR, Valleau JC, Barling AN, Franco JG, DeCapo M, Bagley JL, et al. Exposure to a high-fat diet during early development programs behavior and impairs the central serotonergic system in juvenile non-human primates. Front Endocrinol (Lausanne). 2017;8.
Wurzman R, Forcelli PA, Griffey CJ, Kromer LF. Repetitive grooming and sensorimotor abnormalities in an ephrin-A knockout model for Autism Spectrum Disorders. Behav Brain Res. 2015. 2015. https://doi.org/10.1016/j.bbr.2014.09.012.
Shin Yim Y, Park A, Berrios J, Lafourcade M, Pascual LM, Soares N, et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature. 2017:1–24.
Fernández de Cossío L, Guzmán A, van der Veldt S, Luheshi GN. Prenatal infection leads to ASD-like b ehavior and altered synaptic pruning in the mouse offspring. Brain Behav Immun. 2017;63:88–98.
Manitz MP, Plümper J, Demir S, Ahrens M, Eßlinger M, Wachholz S et al (2016) Flow cytometric characterization of microglia in the offspring of PolyI: C treated mice. Brain Res 1636:172–182
Mattei D, Ivanov A, Ferrai C, Jordan P, Guneykaya D, Buonfiglioli A, et al. Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl Psychiatry. 2017;7:e1120–e1120.51.
Zerbo O, Yoshida C, Grether JK, Van de Water J, Ashwood P, Delorenze GN, et al. Neonatal cytokines and chemokines and risk of autism spectrum disorder: the early markers for autism (EMA) study: A case-control study. J Neuroinflammation. 2014. 2014. https://doi.org/10.1186/1742-2094-11-113.
Marsland AL, Gianaros PJ, Kuan DC-H, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. 2015;48:195–204.
Schrepf A, Kaplan CM, Ichesco E, Larkin T, Harte SE, Harris RE et al (2018) A multi-modal MRI study of the central response to inflammation in rheumatoid arthritis. Nat Commun 9:2243
Trettel F, Di Castro MA, Limatola C (2020) Chemokines: key molecules that orchestrate communication among neurons, microglia and astrocytes to preserve brain function. Neuroscience 439:230–240. https://doi.org/10.1016/j.neuroscience.2019.07.035
Carr MW, Roth SJ, Luther E, Rose SS, Springer TA (1994) Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A 91(9):3652–3656. https://doi.org/10.1073/pnas.91.9.3652
Dong N, Chang L, Wang B, Chu L. Retinal neuronal MCP-1 induced by AGEs stimulates TNF-α expression in rat microglia via p38, ERK, and NF-κB pathways. Mol Vis. 2014. 2014.
Yang Z, Wang J, Yu Y, Li Z. Gene silencing of MCP-1 prevents microglial activation and inflammatory injury after intracerebral hemorrhage. Int Immunopharmacol. 2016. 2016. https://doi.org/10.1016/j.intimp.2016.01.016.
Tian DS, Peng J, Murugan M, Feng LJ, Liu JL, Eyo UB, et al. Chemokine CCL2-CCR2 signaling induces neuronal cell death via STAT3 activation and IL-1β production after status epilepticus. J Neurosci. 2017. 2017. https://doi.org/10.1523/JNEUROSCI.0315-17.2017.
Cui J, Shipley FB, Shannon ML, Alturkistani O, Dani N, Webb MD et al (2020) Inflammation of the Embryonic Choroid Plexus Barrier following Maternal Immune Activation. Dev Cell 55:617-628.e6
Dimitrios D, Akassoglou K (2012) In vivo imaging of the mouse spinal cord using two-photon microscopy. J Vis Exp 59:e2760. https://doi.org/10.3791/2760
Potter LE, Paylor JW, Suh SJ, Tenorio G, Caliaperumal J, Colbourne F et al (2016) Altered excitatory-inhibitory balance within somatosensory cortex is associated with enhanced plasticity and pain sensitivity in a mouse model of multiple sclerosis. J Neuroinflammation 13(1):142. https://doi.org/10.1186/s12974-016-0609-4
Kalambogias J, Chen CC, Khan S, Son T, Wercberger R, Headlam C et al (2020) Development and sensory experience dependent regulation of microglia in barrel cortex. J Comp Neurol 528(4):559–573. https://doi.org/10.1002/cne.24771
Yamamoto M, Horiba M, Buescher JL, Huang D, Gendelman HE, Ransohoff RM et al (2005) Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am J Pathol 166(5):1475–1485. https://doi.org/10.1016/s0002-9440(10)62364-4
Karperien A, Ahammer H, Jelinek HF (2013) Quantitating the subtleties of microglial morphology with fractal analysis. Front Cell Neurosci 7:3. https://doi.org/10.3389/fncel.2013.00003
Fernández-Arjona M, Grondona JM, Granados-Durán P, Fernández-Llebrez P, López-Ávalos MD. Microglia morphological categorization in a rat model of neuroinflammation by hierarchical cluster and principal components analysis. Front Cell Neurosci. 2011. 11:235. doi: https://doi.org/10.3389/fncel.2017.00235. eCollection 2017.
Verdonk F, Roux P, Flamant P, Fiette L, Bozza FA, Simard S et al (2016) Phenotypic clustering: a novel method for microglial morphology analysis. J Neuroinflammation 13(1):153. https://doi.org/10.1186/s12974-016-0614-7
Funding
This work was supported by the National Council of Science and Technology in Mexico (CONACYT) (Grant number: 255317), 708452 CONACYT for L. J. Montalvo-Martínez, 573686 CONACYT for R. Maldonado-Ruiz, and PAICYT 2020 and IBRO-LARC 2020 for Alberto Camacho-Morales.
Author information
Authors and Affiliations
Contributions
RMR, LATV, LMM, OFMG, and VAA performed the experiments. RMR, LATV, LMM, OFMG, VAA, LGO, ROL, EAGV, RGG, and ACM designed and analyzed the experiments. RMR, LATV, LMM, OFMG, VAA, LGO, ROL, EAGV, RGG, and ACM wrote the original manuscript. All authors reviewed, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animal experiments were performed according to the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised in 1996). We followed the Basel Declaration to implement the ethical principles of Replacement, Reduction, and Refinement of experimental animal models. Our study was approved by the local Animal Care Committee (BI20-00004) at the Universidad Autónoma de Nuevo León, Mexico.
Consent to Participate
This study does not include human subjects.
Consent for Publication
This study does not include human subjects.
Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maldonado-Ruiz, R., Trujillo-Villarreal, L.A., Montalvo-Martínez, L. et al. MCP-1 Signaling Disrupts Social Behavior by Modulating Brain Volumetric Changes and Microglia Morphology. Mol Neurobiol 59, 932–949 (2022). https://doi.org/10.1007/s12035-021-02649-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02649-7